Problems: Lumped parameter systems
advertisement
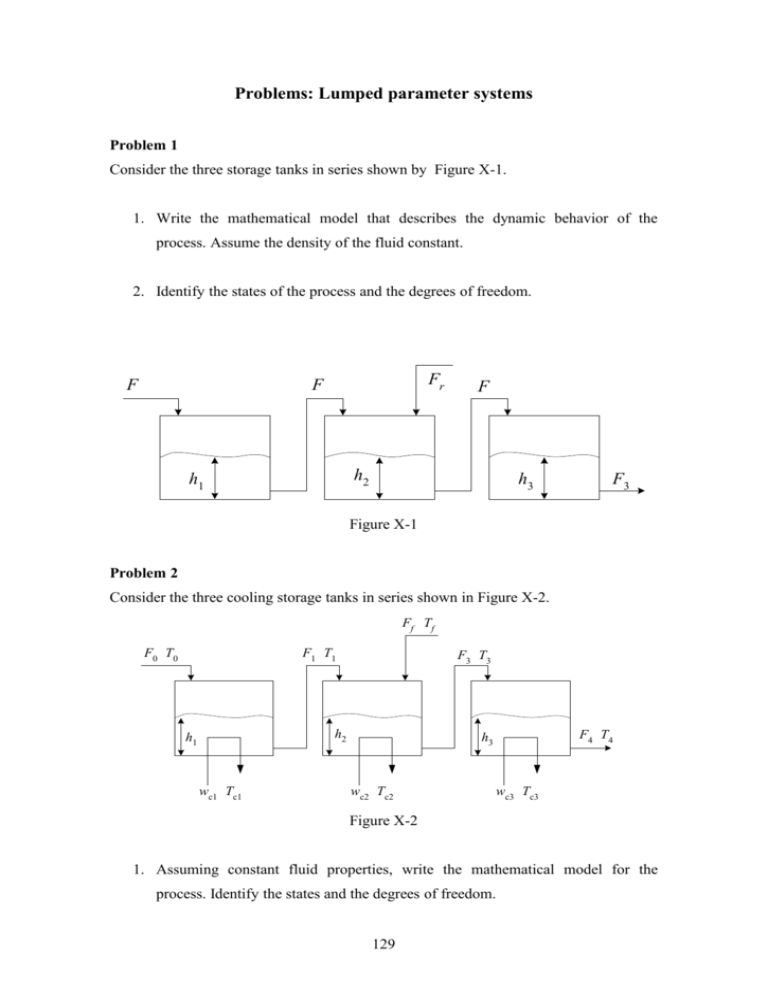
Problems: Lumped parameter systems Problem 1 Consider the three storage tanks in series shown by Figure X-1. 1. Write the mathematical model that describes the dynamic behavior of the process. Assume the density of the fluid constant. 2. Identify the states of the process and the degrees of freedom. F Fr F F h2 h1 h3 F3 Figure X-1 Problem 2 Consider the three cooling storage tanks in series shown in Figure X-2. Ff Tf F0 T0 F1 T1 F3 T3 h2 h1 wc1 Tc1 F4 T4 h3 wc2 Tc2 wc3 Tc3 Figure X-2 1. Assuming constant fluid properties, write the mathematical model for the process. Identify the states and the degrees of freedom. 129 2. Assume now the following non-isothermal reversible first-order liquid-phase reaction: A B is taking place in the three above CSTR in series. Assuming all CSTRs are adiabatic, write down the unsteady state model for the process if the holdups is kept constant in all reactors. Problem 3 Consider the well-stirred non-isothermal reactor shown in Figure X-3 below. Write down the mathematical equations that describe the dynamic behavior of the fundamental quantities of the process. Consider an exothermic liquid-phase second order reaction rate in the form of A B is taking place. The density is assumed to be constant, develop the necessary equations describing the process dynamic behavior. F CA1 h1 F CA3 Figure X-3 Problem 4 Consider a two CSTRs in series with an intermediate mixer introducing a second feed as shown in Figure X-4. A first order irreversible exothermic reaction: A B is carrier out in the process. Water at ambient temperature (Tc1i and Tc2i) is used to cool the reactors. The densities and heat capacities are assumed to be constant and independent of temperature and concentration. Develop the necessary equations describing the process dynamic behavior. Note that the mixer has negligible dynamics and that the inlet feed to CSTR2 has the same temperature as that of the outlet of CSTR1. 130 Q1, C1f, T1f QC1, TC1 Q3, C3, T3 QC1, TC1i Q2, C2, T2 Mixer QC2, TC2 CSTR 1 QC2, TC2i Q4, C4, T4 CSTR 2 Figure X-4 Problem 5: Consider a thermometer bulb with pocket is immersed in hot fluid of temperature T1 as shown in Figure X-5 below. The pocket temperature is T2 (assumed uniform through the thickness) and that for the bulb is T3. Write the model equation that describes how T2 and T3 varies with time. T1 T2 T3 Figure X-5 Problem 6 Consider the single-effect steam evaporator shown in Figure X-6. A salty water (brine) with mass fraction Cb0 and mass flow rate B0 is fed to the evaporator where it is heated with saturated steam with mass flow rate W. The concentrated product comes out of the 131 evaporator with mass flow rate B1 and mass fraction Cb1 while the vapor is withdrawn from the top with mass flow rate V. Develop the dynamic model that describe the process behavior. Assume the heat supplied by the steam is mainly equal to the heat used for vaporizing the brine. Vapor Steam Condensate Feed Concentrated liquid Figure x-6 Problem 7 Consider the process shown in Figure X-7. A stream of pure component A is mixed with another stream of a mixture of component A and B in an adiabatic well stirred mixing tank. The effluent is fed into an adiabatic CSTR where the following reaction takes place: A + B C. Assume the process is isothermal. Develop the dynamic model for the process and determine the degrees of freedom assuming constant fluid properties. Q CA1 Q2 CA2 Q3 CA3 Q4 CA4 Figure X-7 132 Problem 8: Consider the following irreversible first-order liquid-phase reaction: k1 k2 A B C where k1 and k2 are the reaction rate constants in sec-1. 1. Write down the unsteady state model for the process if the reaction takes place in an isothermal well-mixed CSTR. 2. Write down the unsteady state model for the process if the reaction takes place in a non-isothermal well-stirred batch reactor, where the heat needed for the endothermic reaction is supplied through electrical coil. 3. Repeat part 2 but assuming that the reactor is cooled by cold fluid flowing in a jacket and that the reactor wall has high resistance to heat transfer. Problem 9: A perfectly mixed non-isothermal adiabatic reactor carries out a simple first-order exothermic reaction, A B in the liquid phase. The product from the reactor is cooled from the output temperature T to Tc and then introduced to a separation unit where the un-reacted A is separated from the product B. The feed to the separation unit is split into two equal parts top product and bottom product. The bottom product from the separation unit contains 95% of the un-reacted A in the effluent of the reactor and 1% of B in the same stream. The bottom product which is at Temperature Tc (since the separation unit is isothermal) is recycled and mixed with the fresh feed of the reactor and the mixed stream is heated to temperature Tf before being introduced to the reactor. Write the steady state mass and energy balances for the whole process assuming constant physical properties and heat of reaction. Discuss also the degree of freedom of the resulting model. The process is depicted in figure X-8 133 V= 0.5 F CSTR F, Tf C A, C B , T c Separator F Fo, To ,CAo L = 0.5 F Figure X-8 Problem 10: Consider a biological reactor with recycle usually used for wastewater treatment as depicted in figure X-9 below. Substrate and biomass are fed to the reactor with concentrations Sf and Xf. The effluent form the well-mixed reactor is settled in a clarifier and a portion of the concentrated sludge is returned to the reactor with flow rate Qr and Xr. If the reaction of substrate in the clarifier is negligible, the recycle stream would contain the same substrate concentration as the effluent from the reactor. Sludge is withdrawn directly from the reactor with a fraction W. Assuming constant holdup develop the dynamic model for the process. Assume the rate of disappearance of S is given by: r = mS/(K + S) and the rate of generation of X by r/Y where Y is constant. W X S Q Xf Sf Reactor V Settler X S Qr , Xr , S Figure X-9 134 Q-W Xt S Problem 11 Consider the two phase reactor and a condenser with recycle. Gaseous A and liquid B enters the reactor at flow rates FA and FB respectively. Gas A diffuses into the liquid phase where it reacts with B producing C. The latter diffuses into the vapor phase where B is nonvolatile. The vapor phase is fed to the condenser where the un-reacted A is cooled and the condensate is recycled back to the reactor. The product C is withdrawn with the vapor leaving the condenser. For the given information develop the dynamic model for the process shown in Figure X-10. Consider all flows are in moles. F1v, yA1, yc1 T2 P2 T1, P1 FA, TA FB, TB NA NA F2L,xA2 NC Q1 A + B = 2C F1L, xA1, xB1, T1 Figure X-10 Problem 12: Develop the mathematical model for the triple-effect evaporator system shown in figure X-11 below. Assume boiling point elevations are negligible and that the effect of composition on liquid enthalpy is neglected. 135 V1=F-L1 V2=L1-L2 Vapor V3=L2-L3 Vapor Vapor F (feed) Vo Vo V1 L1 V2 L2 Thick Liquor Thick Liquor L3 Thick Liquor Figure X-11 Problem 13 Liquid vaporizer as depicted in Figure X-12 below is one of the important processing units in a chemical plant. A liquid feed, enters the vaporizer at specific flow rate F0 density 0 and temperature T0. Inside the vessel, the liquid is vaporized by continuous heating using hot oil. The mass rate of vaporization is wn. The formed vapor is withdrawn continuously from the top of the vessel. We would like to develop a mathematical model to describe the process. Unlike the adiabatic flash operation, the temperature and pressure in the two phases are different. Correspondingly, the volume of the two phases varies with time. Fv Pv , Vv , v wv Fo , To , o PL, VL, L Q Figure X-12 136 Problems: Distributed Parameter Systems Problem 14 At time t = 0 a billet of mass M, surface area A, and Temperature To is dropped into a tank of water at Temperature Tw. Assume that the heat-transfer coefficient between the billet and water is h. If the mass of water is large enough that its temperature is virtually constant, determine the equation describing the variation of billet temperature with time for the following two cases: 1. The thermal conductivity of the metal in the billet is sufficiently high that the billet is of uniform temperature. 2. The thermal conductivity of the metal is low and the billet radius is large enough that the temperature inside the billet is not uniform. Problem 15 (a)Consider a wall made up of stacked layers of various materials each with different thermal conductivity usually used for insulation as shown by figure X-13a. Assume the inner side is at high temperature Tb and the outer side is at the ambient temperature Ta. Derive the temperature profile through the wall. Tb Ta x Figure X-13a (b) Repeat the above development for a composite cylindrical wall shown by figure X13b. 137 r Tb Ta Figure X-13b Problem 16 A chemical reaction is being carried out in a fixed bed flow reactor. The reaction zone is filled with catalyst pellets. Assume plug flow and the reactor wall is well insulated such that the temperature is uniform in the radial direction. If the fluid enters the reactor with temperature T1 and superficial velocity v and that thermal energy per unit volume (S) is produced inside the reactor due to chemical reaction, derive the steady-state temperature axial distribution. The unit is depicted in figure X-14. Insulating wall Reactant r z=0 Catalyst particles Product z Reaction zone z=L Figure X-14 Problem 17 A first order irreversible reaction is taking place in a tubular reactor. The reactor can be considered to be isothermal and radial dispersion is not to be neglected. Using Fick’s law with effective diffusion coefficient to describe the axial and radial dispersion, derive the steady state mass balance equation and its boundary conditions. 138 Problem 18 (a) Derive the unsteady state mass balance equation for the diffusion of species A through a spherical porous pellet. (b) Derive the steady state mass balance equation for the diffusion of species A through a spherical porous pellet where it converts to B according to the first-order reaction: AB. Problem 19 Species A dissolves in liquid B and diffuses into stagnant liquid phase contained in a cylindrical tank where it undergoes a homogeneous irreversible second-order chemical reaction: A+B C. Derive the mass balance equation for component A. Assume that the depth of liquid is so large compared to the radius of the tank, thus, the radial distribution can be neglected. The process is shown schematically in figure X-15. Gas A z=0 Liquid B Dz z=L Figure X-15 139 129








