Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical
advertisement

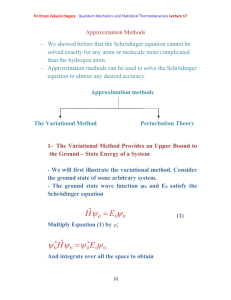
Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture 21 Statistical Thermodynamics Introduction and Definitions Statistical Thermodynamics: is the application of probability theory, which includes mathematical tools for dealing with large populations, to the field of mechanics, which is concerned with the motion of particles when subjected to a force. - It provides a framework for relating the microscopic properties of individual atoms and molecules to the macroscopic or bulk properties of materials that can be observed in everyday life, therefore explaining thermodynamics as a natural result of statistics and mechanics (classical and quantum) at the microscopic level. - It provides a molecular-level interpretation of thermodynamic quantities such as work, heat, free energy, and entropy, allowing the thermodynamic properties of bulk materials to be related to the spectroscopic data of individual molecules. - Statistical thermodynamics was born in 1870 with the work of Austrian physicist Ludwig Boltzmann, much of which was collectively published in Boltzmann's 1896 Lectures on Gas Theory.[2] Boltzmann's original papers on the statistical interpretation of thermodynamics, the H-theorem, transport theory, thermal equilibrium, the equation of state of gases, and similar subjects, occupy about 2,000 pages in the proceedings of the Vienna Academy and other societies. The term "statistical thermodynamics" was proposed for use by the American thermodynamicist and physical chemist J. Willard Gibbs in 1902. According to Gibbs, the term "statistical", in the context of mechanics, i.e. statistical mechanics, was first used by the Scottish physicist James Clerk Maxwell in 1871. [1] Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture 21 Some Definitions must be taken in statistical thermodynamics such as: First law of thermodynamics: states that energy can be transformed (changed from one form to another), but it can neither be created nor destroyed. Heat is a process by which energy is either added to a system from a high-temperature source or lost from a system to a low-temperature sink energy may be lost by the system when it does work on its surroundings, or conversely, energy may be gained as a result of work done to it by its surroundings The internal energy of a thermodynamic system, or a body with well-defined boundaries, denoted by U, or sometimes E, is the total of the kinetic energy due to the motion of molecules (translational, rotational, vibrational) and the potential energy associated with the vibrational and electric energy of atoms within molecules or crystals. It includes the energy in all of the chemical bonds, and the energy of the free, conduction electrons in metals. - The increase in the internal energy of a system is equal to the amount of energy added by heating the system, minus the amount lost as a result of the work done by the system on its surroundings. The first law can be stated mathematically as: where dU is a small change in the internal energy of the system, δQ is a small amount of heat added to the system, and δW is a small amount of work done by the system. The sign convention here is that δQ < 0 if energy is lost from the system as heat, but δW > 0 if energy is lost from the system as work. Note that some textbooks (e.g., Greiner Neise Stocker) alter the sign convention for W and formulate the first law as: [2] Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture 21 where δW is the work done on the system. This amounts to again using δQ < 0 if energy leaves the system as heat, but now taking δW < 0 if energy leaves the system as work.[1] So, when a system (e.g., a gas) expands the work done − PdV whereas in the previous formulation of the first law, the work done by the gas while expanding is PdV. In any case, both give the same result when written explicitly as: In other words δw = PdV where P is pressure and V is volume. Also, for a reversible process, the total amount of heat added to a system can be expressed as δQ = TdS where T is temperature and S is entropy. Therefore, for a reversible process, : The second law of thermodynamics is an expression of the universal law of increasing entropy, stating that the entropy of an isolated system which is not in equilibrium will tend to increase over time, approaching a maximum value at equilibrium. In statistical thermodynamics, entropy is defined as Statistical mechanics explains entropy as the amount of uncertainty which remains about a system, after its observable macroscopic properties have been taken into account. For a given set of macroscopic variables, like temperature and volume, the entropy measures the degree to which the probability of the system is spread out over different possible quantum states. The more states available to the system with appreciable probability, the greater the entropy. More specifically, entropy is a logarithmic measure of the density of states. In essence, the most general interpretation of entropy is as a measure of our uncertainty about a system. The equilibrium state of a system [3] Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture 21 maximizes the entropy because we have lost all information about the initial conditions except for the conserved variables; maximizing the entropy maximizes our ignorance about the details of the system.[6] This uncertainty is not of the everyday subjective kind, but rather the uncertainty inherent to the experimental method and interpretative model. where kB is Boltzmann's constant 1.38066×10−23 J K−1 and is the number of microstates corresponding to the observed thermodynamic macrostate. A common mistake is taking this formula as a hard general definition of entropy. This equation is valid only if each microstate is equally accessible (each microstate has an equal probability of occurring). Boltzmann Distribution If the system is large the Boltzmann distribution could be used (the Boltzmann distribution is an approximate result) This can now be used with : [4] Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical Thermodynamics Lecture 21 [5]