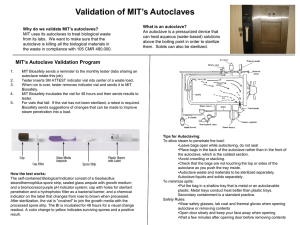
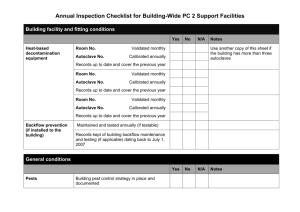
1103 - Academic lab pages
advertisement

Microorganisms of various hazard groups contained on solid media which may also contain hazardous substances,Autoclave controls consisting of,3M Attest vials containing Liqiud media , crushed glass vial and Bacillus stearothermophilus spores and culture,Thermalog S chemical indicator check strip. BIOHAZARD.TOXIC.CARCINOGENIC.SENSITIZING AGENTS.3M ATTEST INDICATORS ,TINY GLASS VIALS ARE CONTAINED IN THE PLASTIC OUTER CONTAINER. As well as the biological hazard from microoganisms some of the substances incorporated in solid media may be Toxic,carcinogenic and sensitizing agents. 5 INTENDED USE Hazardous Substances Policy - Assessment CHEMICAL HAZARD AND RISK ASSESSMENT School of Biosciences Name of supervisor Dr.T.W.Young Assessment Number* 1103 Date of Assessment 04/01/01 Signature Assessor A.Wadeson Signature Notes A School COSHH form in Word is available on the School Server. Available from the Health and Safety Unit. Guidance on making an assessment is given in Making a Chemical Hazard and Risk Assessment. Guidance is also available from Guidance on Completing the Chemical Hazard and Risk Assessment Form. Use a continuation sheet to expand any section of this form in hard copy version. 1 LOCATION OF THE WORK ACTIVITY 2 PERSONS WHO MAY BE AT RISK G6/G7 List names where possible Dr T,W.Young,A.Wadeson and all other members of Dr T.W.Young's research team. Final year project students.C.Webster,L Griffiths,R.Godfrey and all personnel who may use the autoclave for safe discard of the Ground floor communal microbiological waste. 3 ACTIVITY ASSESSED 4 MATERIALS INVOLVED NAME Sterilisation of microbiological waste,Petri dishes and autoclave controls using the Labclave 007 autoclave in laboratory G7. AMOUNT** Microorganisms of various hazard groups contained on solid media which may also contain hazardous substances, Autoclave controls consisting of,3M Attest vials containing Liqiud media , crushed glass vial and Bacillus stearothermophilus spores and culture,Thermalog S chemical indicator check strip. 5 HAZARD BIOHAZARD. TOXIC. CARCINOGENIC. SENSITIZING AGENTS. RISK PHRASES HAZDAT NO*** BIOSCIENCESNO*** As well as the biological hazard from microoganisms some of the substances incorporated in solid media may be Toxic,carcinogenic and sensitizing agents. 3M ATTEST INDICATORS , TINY GLASS VIALS ARE CONTAINED IN THE PLASTIC OUTER CONTAINER. INTENDED USE Give brief details and attach protocol/instructions 6 Attach copies of data sheet(s) Sterilisation of waste petri dishes containing microorganisms on solid media,sterilisation of autoclave controls prior to incubation and after incubation and sterilisation of control samples of Bacillus stearothermophilus autoclave controls. Sterilisation af Thermalog S chemical indicator strips. RISKS to HEALTH and SAFETY from INTENDED USE From personal exposure or hazardous reactions. Refer to OELs, flash points, etc., as appropriate. Are pregnant women, breast-feeding mothers especially at risk? Risk of skin and eye contact with microbiological waste,media components and additions,some of which may be hazardous.Risk of burns from from hot containers and liquids,risk of bursting of 3M Attest vials and flying bebris including broken glass. RISK OF CONTACT WITH UNSTERILE MICROBIOLOGICAL WASTE UNTIL AUTOCLAVE CONTROLS HAVE BEEN VERIFIED. 7 CONCLUSIONS ABOUT RISKS Is level of risk acceptable? Can risk be prevented or reduced by change of substance/procedure? Are control measures necessary? The risks in section 6 above are significant ,they should be minimised by using Good Microbiological Practice together with the precautions and instructions given by 3M health care with the Attest vials. The precautions given in section 8 should be followed. 8 CONTROL MEASURES Additional to Good Chemical Practice Face shield should be used to prevent contact with hot waste and flying debris including broken glass vials. Follow instructions given with attest vials. When the Attest vials are cool and a vial crusher is not available ,the vial can be wrapped in a cloth and a pair of plyers or forceps used to gently exert pressure on the vial to break it, prior to incubation. Microbiological waste, which should always be cool enough to handle safely, should always be handled wearing disposable gloves at all times. Autoclave gloves should be worn for added safety during glass vial rupture. Ensure adequate ventilation by using the window fan. DO NOT HANDLE UNSTERILE MICROBIOLOGIACL WASTE OR CONTROLS WITHOUT GLOVES. Glovesused during the procedure should be put for subsequent sterilisation. 9 INSTRUCTION/TRAINING Specify course(s) and/or special arrangements. Check integrity of gloves, both disposable and autoclave types. Check face shield is good order and clean. 10 MONITORING Performance of control measures, Personal exposure Health Surveillance 11 WASTE DISPOSAL PROCEDURE See School Server for Approved Procedure Document on specific Chemical Waste Disposal. Ensure waste agar is washed away with copious amounts of warm water to prevent waste agar solidifying in drains.Incubated controls, positive, showing growth and negative, showing no growth, should be added to the waste petri dishes before autoclaving and disposed of in the rest of the plastic mass of waste. 12 REVIEW Enter the date or circumstances for review of assessment (maximum review interval 5 years) 04/01/06 or Any change in the protocol. 13 EMERGENCY ACTION TO CONTROL HAZARDS To stabilize situation eg spread absorbant on liquid spill; eliminate sources of ignition, etc. Spills of unsterilised microbiological waste should be mopped up with presept granules wearing face shield and disposable gloves. The same procedure should be used for sterilised waste as the results of the autoclave controls will not be known until after incubation. Gloves from this procedure should be put for subsequent sterilisation. Evacuation, protection for personnel involved in clean-up, Special First Aid TO PROTECT PERSONNEL ONLY PERSONNEL WHO ARE ADEQUATELY TRAINED SHOULD ATTEMPT TO CLEAR UP SPILLAGES. Clean-up/decontamination TO RENDER SITE OF EMERGENCY SAFE After cleaning up spill,wash site with soap and water. CONTACT A.Wadeson or First aider. PHONE 3523 or see first aider list 10.10.00 * Prefix T is used for Teaching Assessment Number. ** List the amount by weight of the substance used. (for liquids eg; 100 mls 1M Sodium Hydroxide = 4g). *** Hazdat No is the UNICOSHH datasheet report number. Biosciences No is the Biosciences data sheet number. UNICOSHH IS A CHEMICAL DATABASE ON THE HEALTH AND SAFETY UNIT SERVER. BIOSCIENCES DATA SHEETS ARE AVAILABLE IN THE SCHOOL SAFETY OFFICE.