Acids and Metals Summary Notes
advertisement
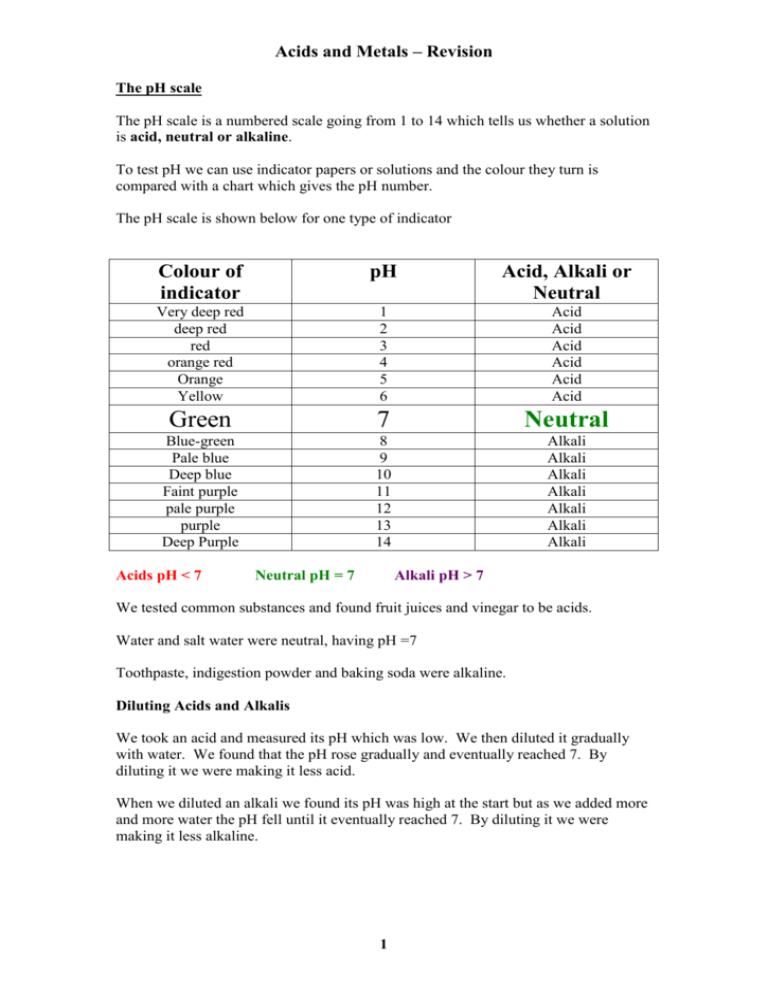
Acids and Metals – Revision The pH scale The pH scale is a numbered scale going from 1 to 14 which tells us whether a solution is acid, neutral or alkaline. To test pH we can use indicator papers or solutions and the colour they turn is compared with a chart which gives the pH number. The pH scale is shown below for one type of indicator Colour of indicator pH Acid, Alkali or Neutral Very deep red deep red red orange red Orange Yellow 1 2 3 4 5 6 Acid Acid Acid Acid Acid Acid Green 7 Neutral Blue-green Pale blue Deep blue Faint purple pale purple purple Deep Purple 8 9 10 11 12 13 14 Alkali Alkali Alkali Alkali Alkali Alkali Alkali Acids pH < 7 Neutral pH = 7 Alkali pH > 7 We tested common substances and found fruit juices and vinegar to be acids. Water and salt water were neutral, having pH =7 Toothpaste, indigestion powder and baking soda were alkaline. Diluting Acids and Alkalis We took an acid and measured its pH which was low. We then diluted it gradually with water. We found that the pH rose gradually and eventually reached 7. By diluting it we were making it less acid. When we diluted an alkali we found its pH was high at the start but as we added more and more water the pH fell until it eventually reached 7. By diluting it we were making it less alkaline. 1 Acids and Metals – Revision Neutralisation We carried out a number of experiments where we added acid gradually to alkali with Universal indicator present so that the pH could be checked. We found that the acid gradually cancelled out or neutralised the alkali. We did the same experiment in reverse adding the alkali to the acid and found that the alkali neutralised the acid. When we take an indigestion tablet we are trying to cure a problem caused by too much acid in our stomachs. The indigestion tablet contains an alkali which gradually cancels out or neutralises some of the acid in our stomach making us more comfortable. Bee stings are alkaline and can be relieved by using vinegar which is an acid to neutralise the alkali. The Reactivity Series We carried out a number of experiments with metals and found that some are more reactive than others. When the metals are put in a league table in order of reactivity it looks like this: Potassium Sodium Calcium Magnesium Aluminium Zinc Iron Nickel Tin Lead Copper Mercury Silver Gold Platinum Most reactive – react easily with cold water. The MAZINTL metals react with dilute acid Very unreactive – won’t even react with acid Uses of Metals Un-reactive metals like copper are suitable for use as water pipes because they won’t react with or dissolve in the water. Gold and platinum do not react with air, water or chemicals in the home so can be used as jewellery. They keep a nice clean shiny appearance. 2 Acids and Metals – Revision Getting Metals from the Earth Since gold is un-reactive it can be found as the pure metal in the earth – it does not react with its surroundings like potassium, calcium and magnesium would. Iron is found on the earth in a substance called iron ore. To get the iron from the ore takes a lot of heat energy. Iron is separated from its ore in a furnace called the Blast Furnace. It is heated with coke and limestone. Molten iron is produced as well as a waste product called slag. Potassium, sodium and calcium are very reactive metals and are among the latest metals to be discovered. Only relatively recently has man had the technology to get these metal out of their ores. Displacement We carried out some experiments in which a reactive metal reacted with a solution containing another metal. For example magnesium reacted with blue copper sulphate solution. The solution became gradually less blue and small pieces of copper appeared at the bottom. The magnesium began to dissolve. The reactive magnesium had displaced (pushed out) the less reactive copper from its solution. A metal can displace a metal lower in the Reactivity Series from its compound. Electrolysis We passed an electric current through copper chloride solution (CuCl2). Copper metal stuck to the negative rod and bubbles of chlorine gas (smell of bleach) appeared at the positive rod. The electrical energy had broken the compound to give the metal Electroplating We used electricity to cover a piece of copper in nickel. This process is called electroplating. It can be used to improve the appearance of cheaper metals by plating them with precious metals. This means that gold plated jewellery is more affordable than jewellery containing mostly gold. 3