Worksheet for Video #2 - Reactivity of Metals: Li, Na, and K
advertisement

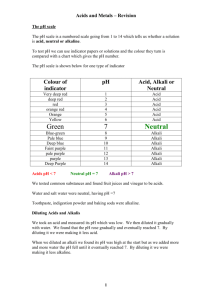
Worksheet for Video #2 - Reactivity of Metals: Li, Na, and K Complete this page after viewing the video and performing the investigation. 1. What is the educational purpose of demonstrating the reactivity of the alkali metals? 2. Why is it necessary to keep the quantity of alkali metal in the classroom to a minimum? 3. Why is it essential for all equipment that comes in contact with the alkali metal samples to be coated with mineral oil? 4. Why is lithium safer for students to use than potassium? 5. Why is a safety shield required for the demonstration? 6. Why is phenolphthalein added to the water? 7. How will the activity change if phenolphthalein is not added? 8. Why is a wide-mouth container like a beaker preferable to a narrow-mouth container like an Erlenmeyer flask or test tube for this investigation? 9. Why is a wire mesh placed above the beaker in which the alkali metal reacts with water? 10. Explain why leftover pieces of metal must NOT be discarded in the trash or the sink. 11. What are possible fire hazards when working with the alkali metals? 12. Observation charts can be developed by students or created by teachers. How does each method reflect different student skill levels for this investigation? Answers - Reactivity of Metals: Li, Na, and K Complete this page after viewing the video and performing the investigation. 1. What is the educational purpose of demonstrating the reactivity of the alkali metals? The reactivity of the alkali metals increases as you descend the group I elements of the periodic table. 2. Why is it necessary to keep the quantity of alkali metal in the classroom to a minimum? Alkali metals are light, flammable metals that react vigorously with water to produce hydrogen gas. A pea-size sample is appropriate for a teacher demonstration but too large for a student investigation. Do not be tempted to use larger pieces of these metals to produce larger and more dramatic flames. The reaction, particularly with potassium may become too unpredictable. 3. Why is it essential for all equipment that comes in contact with the alkali metal samples to be coated with mineral oil? Alkali metals react vigorously with water so it is important to ensure that they do not come into contact with possible sources of water. Cut each metal sample on a tray with oil to prevent accidental contact with water. 4. Why is lithium safer for students to use than potassium? Lithium reacts less vigorously with water than either sodium or potassium to produce less heat energy, so the risk of creating a hydrogen gas explosion is greatly reduced. 5. Why is a safety shield required for the demonstration? Sodium and potassium react vigorously with water to create a lot of heat energy. As a result, the risk of hydrogen gas exploding is greater. This means that a small piece of metal can be raised out of the water from the explosion. Moving as a projectile, these pieces of metal can land on a person or object and continue to react. Very rarely, glass can break and the shield offers protection from broken glass. 6. Why is phenolphthalein added to the water? Phenolphthalein indicator provides a visible trail of the moving metal. In the Grade 11 Chemistry University course this pink trail is also evidence of the formation of a base as a product of the reaction. 7. How will the activity change if phenolphthalein is not added? The alkali metal reaction with water will not change, but the trail showing the movement of the metal will not be visible. As a demonstration, this means that some students may have more difficulty observing the action of the metal in the water. 8. Why is a wide-mouth container like a beaker preferable to a narrow-mouth container like an Erlenmeyer flask or test tube for this investigation? Open beakers allow the hydrogen gas to dissipate more easily. More constrained containers will concentrate the gas and the heat of reaction is then more likely to cause the hydrogen gas to combust and possibly lead to a fire or an explosion. 9. Why is a wire mesh placed above the beaker in which the alkali metal reacts with water? A square wire mesh is placed over the mouth of the beaker to prevent the alkali metals from being ejected from the beaker. 10. Explain why leftover pieces of metal must NOT be discarded in the trash or the sink. These metals are flammable materials. They will react with water to release energy that can lead to a fire or an explosion. 11. What are possible fire hazards when working with the alkali metals? Alkali metals react vigorously with water to create hydrogen gas. The heat of reaction with sodium and potassium is high enough to ignite the gas and cause an explosion. A small explosion can push the metal out of the container so that it can land on a person or combustible material and continue to react. Keep all samples of the alkali metals in mineral oil until they are to be used to avoid accidental contact with water. 12. Observation charts can be developed by students or created by teachers. How does each method reflect different student skill levels for this investigation? Observation charts created by students require them to understand what they are going to observe and record. Teacher observation charts help students focus on specific aspects of the inquiry to build both their observation skills and recording skills. Students should be exposed to both situations as they reflect different levels of experience and skill development.