ESS 461 U-series dating of marine samples
advertisement

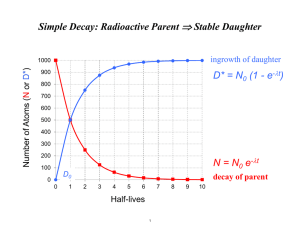
ESS 461 U-series dating of marine samples Under oxidising conditions in seawater U6+ forms the highly soluble uranyl carbonate complex [UO2(CO3)3]4-. By comparison, Th behaves as a highly insoluble element. In reality, it "adsorbs" to the surfaces of fine sediment particles and is rapidly stripped from the water column and transferred to sediment accumulating on the seafloor. Thus the 238U chain is continuously broken at the transition from 234U to 230Th. (1) What effect will this have on the 226Ra and 210Pb activity of seawater compared to 238U ? How quickly after its formation is Th removed from the water column? Fortunately, there are two short-lived isotopes of Th that provide an answer: 238U -> 234Th 228Ra -> 228Th (t1/2 for (t1/2 for 234Th 228Th = 24.1 days; decays to 234Pa) = 1.91 years; decays to 224Ra) Both parents are quite soluble in seawater (Ra behaves similarly to Ba). (2) What would you conclude about the average time required to remove an atom of Th from seawater if the activity ratios ( 234Th / 238U ) and ( 228Th / 228Ra ) were: (a) both << 1 (b) both approximately equal to 1 (c) if ( 234Th / 238U ) ~ 1 and ( 228Th / 228Ra ) << 1 ? The figure below shows a plot of these activity ratios in surface seawater, from which the mean lifetime of Th (against scavenging and removal by particles, not radioactive decay) is estimated to be on the order of 1 year. Note that the ocean surface is where most biological activity occurs and where the density of particles is greatest, causing effective removal of Th. Many particles which settle from out of the upper ocean will re-dissolve before they reach the seafloor, releasing Th back into the water column. The mean lifetime of Th in the deep ocean is ~ 30 years, much longer than in surface and coastal waters. Measurement of long-term sedimentation rates in the deep ocean Seawater contains approximately 3.1 ppb (by weight; i.e. 3.1 x 10-9 g/g) of U with an activity ratio (234U/238U) = 1.15. (3) What is the production rate of 230Th in a column of water 4 km deep and 1 cm2 in crosssectional area ? Take the density of seawater as 1.036 g/cm3. It will be easiest to calculate the production rate in atoms/cm2/yr. Be careful if you express the U and Th in "dpm" equivalents. The molar weight of U is 237.969 g/mol. The atomic abundance of 238U is 99.28% The decay constant of 238U = 1.5513 x 10-10 yr-1 The decay constant of 234U = 2.83 x 10-6 yr-1. This is a good estimate of the flux of 230Th to the seafloor - i.e. the number of atoms per cm2 per year "raining" with sediment onto the seabed (because 230Th is stripped so rapidly from the water column, we can assume that no decay occurs in the time it takes for particles to sink through the few km to the bed). (4) Sediment at the top of a deep ocean core from the site described above is found to have a 230Th activity of 14.05 dpm/g. A U measurement by alpha spectrometry gives activities of 1.75 dpm/g for both 238U and 234U (i.e. 234U is in secular equilibrium with its parent 238U). Take the sediment density to be 2 g/cm3. Assume that U in the sediment is tightly bound in retentive mineral sites (this is non-leachable U locked up in clay and iron oxide minerals). What is the apparent sedimentation rate ? Further measurements down the core give the following activities: Depth interval (cm) (238U) dpm/g (234U) dpm/g (230Th) dpm/g 0-1 1.75 1.75 14.05 5-6 1.75 1.75 12.95 9-10 1.75 1.75 12.0 19-20 1.75 1.75 9.9 29-30 1.75 1.75 8.2 39-40 1.75 1.75 6.9 49-50 1.75 1.75 5.85 59-60 1.75 1.75 5.0 69-70 1.75 1.75 4.35 79-80 1.75 1.75 3.8 (5) The half-life of 230Th is 75,400 years. Estimate the age of the sediment at 30 cm depth. What assumptions are required to calculate an age ? Are they justified in this case ? Over what interval of the core ? (6) Suppose you measured a couple of extra samples from near the top of the core, and got the following results: Depth interval (cm) (238U) dpm/g (234U) dpm/g (230Th) dpm/g 0-1 1.75 1.75 14.05 1-2 1.75 1.75 14.00 2-3 1.75 1.75 14.00 What might explain the constant 230Th activity in the top ~ 3 cm of the core ? How could you test this using 228Th or (perhaps) 234Th ? Qualitatively, how does this affect your estimate of the sedimentation rate in Q. (4)