U-series disequilibrium I
advertisement

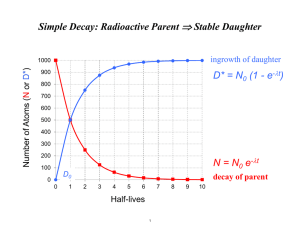
U-series Disequilibrium 1 9/6/12 Lecture outline: Zircon 1) Secular equillibrium and disequilibrium 2) U-Th systematics 3) U-excess 4) U-Th disequilbrium dating 5) 232Th 6) Th excess and sedimentation rates “initial” corrections Geological archives dated by U-series disequilibrium: speleothems (top) and fossil coral terraces (bottom) Secular equilibrium and disequilibrium Secular equilibrium: all radioactive species in a decay chain have the same activity Disequilibrium: system is perturbed (removal/enrichment of daughter/parent), and system decays back to secular equilibrium And if you know (disequilibrium)initial, can calculate t since disequilibrium For U-series decay chain, what are some examples of processes that cause disequilibrium ? There are two types of disequilibria: 1) Daughter excess (i.e. activity of daughter > activity of parent) 2) Daughter deficit (Ad < Ap) Decay Chain Systematics: Consider a 3-member decay chain: l l 1 ® N ¾¾ 2® N N1 ¾¾ 2 3 Evolution of this system is governed by the coupled equations: dN1 = -l1N1 dt dN3 = l2 N2 dt dN2 = l1 N1 - l2 N2 dt Note that at secular equil, dN 2 0 dt N2 (t) = l1 ( ) N1o e- l1t - e - l 2t + N2oe - l 2t l2 - l1 As you can see, the solution of these differential equations is quite complicated (except for N1), so we will derive some equations from the disequilibria of 234U and 230Th. 234U NOTE: everything in this lecture will be activities (A), unless otherwise noted Excess Given: (234U/238U)A of ocean = 1.15 Explanation: [you tell me] The activity of (234U)excess decreases with time: U Ex 234 234 0 U Ex e 234 t And excess 234U corresponds to the 234U not supported by 238U: U 234 And dividing through by 238U activity, we obtain: U ( 238 U 234 0 U 1 238 U A 234 238 U )e 234 t U 234 0 238 U U 234 t e 238 So if you measure 234U/238U, and know (234U/238U)initial can calculate age, for t<secular equilibrium Note that fixed analytical error (±0.5%) yields larger and larger age error bars as you approach secular equilibrium 230Th Deficiency Given: Many U-rich minerals (such as carbonate) precipitated with virtually no Th Explanation: [you tell me] So you grow in 230Th due to decay of 238U and excess 234U (in atom number): 234 1 t 230 234 o t t o o t N 2 (t ) 2 1 N1 e 1 e 2 N2e 2 T hEx And converting to activity, substituting formula for and simplifying, we obtain: 230 Th 230 t (1 e ) 238 U A 230 234 230 230 234 234U , Ex U Ex e 234 e 230 t dividing by 238UA, 0 t U t 234 e 230 ) 1 ( e 238 U A 234 IN THEORY: if you measure (230Th/238U)A, and assume initial (234U/238U)A=1.15, then you can calculate a sample’s age. IN PRACTICE: you measure (230Th/238U) and 234U/238U, and iteratively find an age that satisfies both the measurements made today. You then are calculating also 234U/238U initial. So when/where is the assumption that initial (234U/238U)A=1.15 not a good one? 230Th-234U activity growth lines Development of mass spectrometry techniques enable U-Th ages to be measured to ±1% precisions (Edwards et al., 1987) New MC-ICPMS enable precisions down to ±0.1% secular (Shen et al., 2002) equilibrium For most samples: secular equilibrium Common U-Th series applications: 1. Corals - sea level from fossil terraces - climate reconstruction Edwards et al., 1987 2. Cave Stalagmites - climate reconstruction Cutler et al., 2003 but what happens when good samples get “dirty”? FACT: most geological samples contain some “initial” aka “detrital” aka “nonradiogenic” thorium PROBLEM: mass specs cannot distinguish between “detrital” 230Th and radiogenic 230Th SAVING GRACE: “detrital” thorium mostly 232Th (quasi-”stable” on U/Th disequilibrium timescale) STRATEGY: measure 232Th in samples, correct for detrital 230Th using 230Th/232Th of contaminant BUT how do you estimate 230Th/232Th of contaminant? given: average bulk Earth abundance (230Th/232Th)atom = 4.4e-6 at secular equilibrium (Kaufman, 1993) complication: in most settings this will not apply… (why not?) STRATEGY #1: date samples of known age upper error bar = analytical error + correction using (230Th/232Th)atom of 2.0e-5 lower error bar = analytical error - especially good for corals because you can absolutely date them by counting back annual density bands (Cobb et al., 2003a) -if you can identify a well-dated event in your sample (volcanic eruption from historic record?), you may be able to do this for older samples STRATEGY #2: generate isochrons from samples IDEA: sample multiple samples of the same age but different 232Th concentrations, then you know that they all contain the same 230Thrad, and that 230Thnr will scale with 232Th “dirty” edges “clean” middle “dirty” edges U/Th isochron plots most often used for stalagmites (Partin et al., 2007) in these plots: slope ≈ age intercept = (230Th/232Th)act of contaminant phase Usually huge range of values uncovered… Partin et al., 2007 translating into huge age errors for “dirty” samples LESSON: keep it clean (if possible) 230Th Excess and Deep-Sea sediments Phenomenon: “excess” 230Th present in ocean sediments Explanation? The activity of (230Th)excess decreases with time: 230 T hEx 230 0 T hEx e 230 t We can define sedimentation rate as: S=distance/time; so t=d/S and 230 (d / S ) 230 0 T hEx T hEx e 230 and ln( 230 T h E x ) ln( 230 T hEx ) 0 d S ln(230Th)ex If you assume that delivery of 230ThEx is constant through time, can calculate age as a function of depth - or - sedimentation rate Slope = -/S ( 230 ) Depth in sediment core 230Th Excess and sedimentation rate changes 230Th Excess - interpretation But how can you have an increase of 230Thex with depth, given constant 230Thex delivery? Down-core measurements in Norwegian core show sedimentation rate changes Increased scavenging of 230Th during interglacials in Norway, or sediment focusing. So 230Thex delivery is not constant. Changes in the rain-rate of particles will lead to increases and decreases in Th scavenging. What could change scavenging rate?