SYNTHESIS OF α-ALUMINA FINE POWDERS WITH SEEDING BY
advertisement

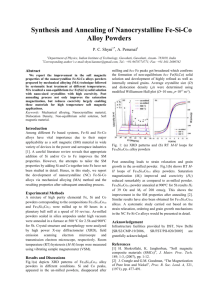
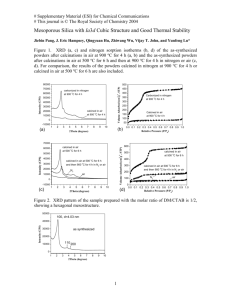
SYNTHESIS OF α-ALUMINA FINE POWDERS WITH SEEDING BY AN EGG WHITE SIMPLE ROUTE R. E. P. Salem1,3*; E. M. Silva Jr.2; A. L. Chinelatto2; A. S. A. Chinelatto2; E. M. J. A. Pallone3 1 Department of Materials Engineering, Paraná Federal University of Technology, Av. Dos Pioneiros, 3131, Londrina, Paraná, Brazil, 86036-360 2 Department of Materials Engineering, Ponta Grossa State University, Av. Gal. Carlos Cavalcanti, 4748, Ponta Grossa, Paraná, Brazil, 84030-900 3 FZEA – São Paulo University, Av. Duque de Caxias Norte, 225, Pirassununga, São Paulo, Brazil, 13635-900 Alumina ceramics are used in many areas of industry because of their excellent mechanical, thermal and optical properties. The synthesis of alumina powders in the stable α-alumina phase generally requires calcination temperatures of above 1200ºC, in order to promote the complete transformation from metastable alumina phases. Many studies have been done trying to reduce temperature of nucleation and growth of α-alumina, intending to produce a stable powder with smaller particle size. In this work, a simplified route using egg white and aluminium nitrate in aqueous medium was used to synthesize powders of ultrafine alumina. A small quantity of submicrometric α-alumina seeds (1% in weight) was added to the gel as an attempt to decrease the energy of activation and, consequently, the temperature of formation of α-alumina. The egg white-based precursor was characterized by differential thermal analysis associated with thermogravimetry and calcined at 700-1200ºC under air. The resulting powders were characterized by X-ray diffraction, scanning electron microscopy and infrared spectroscopy. Results showed that the egg white route was effective to synthesize submicrometric alumina powders, and the seeded powders promoted formation of α-alumina particles at lower calcination temperatures than non-seeded powders. Keywords: α-alumina, synthesis, egg white, seeds, calcination. Introduction Alumina is a ceramic oxide with wide applicability due to its good mechanical properties and high refractoriness. The most stable alumina phase, the α phase, is used based on these properties. The α-alumina produced by conventional process (Bayer process) forms at temperatures near 1200oC, having the morphology of micrometric and submicrometric particle aggregates. However, various chemical routes have been widely studied in order to synthesize α-alumina and other ceramic oxides, aiming to produce a more homogeneous material at lower temperatures, thus preventing particle growth and aggregation and controlling more rigorously the properties of final powders. Recent works [1,2] have shown synthesis of oxides using egg white as precursor medium. Egg white is predominantly composed of water (>80%) and the remainder is composed of proteins and a small amount of sugars and mineral salts. Among the proteins, the one in largest quantity is ovalbumin, which has a molecule with charged sites, in which ions are able to solvate in solution [2]. The main motivation to use egg white is that it has been confirmed as very suitable matrix to uptake of metallic ions. After thermal treatment in relatively low temperatures, it allows the formation of amorphous and crystalline powders, nanometric and submicrometric, in a simple, economical and enviromentally sustainable way. In order to provide low-energy sites for the formation of α-phase at lower temperatures than in the conventional process, seeding of α-alumina particles into the precursor on chemical synthesis has been reported with success [3-4]. The purpose of this work is to study the synthesis of α-alumina with seeding by the egg white route, evaluating phase evolution, morphology and microstructure of the powders, and consequently, the viability of this method for this material. Experimental part Aluminium nitrate nona-hydrate (Al(NO3)3.9H2O, Vetec, Brazil) was dissolved in a small amount of water and dropped in a fresh extracted egg white aqueous solution (60% vol) under magnetic stirring. After 10 minutes, α-alumina seeds (AKP-53, Sumitomo Chemical, Japan) were added to the mixture. The weight of seeds added was 1% of the estimated mass of alumina to be produced. After vigorous stirring for 30 minutes, the mix was kept on hot plate (110oC) for 24 hours and the dry precursor was crushed in agate mortar and calcinated at 500oC in air for 2 hours. The resulting powder was divided in equal parts and calcined in temperatures varying between 700-1200oC, with intervals of 50oC. The precursors and the calcined powders were characterized by X-ray diffraction, differential thermal analysis with thermogravimetry, scanning electron microscopy with elemental analysis by EDX and infrared spectroscopy. Results and discussion Fig. 1 shows DTA/TG curve of seeded egg white/aluminium nitrate precursor after drying on hot plate. These curves were similar to the curves obtained from non-seeded precursor. In thermogravimetry it is observed a continuous mass loss, attaining about 80% at 1300oC. The mass loss was most vigorous under 500oC, corresponding to the elimination of most of the organic matter from the egg white precursor and hydration water of the aluminium salt. The subsequent mass loss is related to residual organic matter slowly released due to the complex morphology of the powder and its changes with phase transformations. The DTA peak at 790.5oC corresponds to the transformation of amorphous alumina to γ-alumina transition phase. In the range of 950-1200oC it is observed a band in the DTA curve, which corresponds to the continuous process of phase transformation from transition aluminas to α-alumina, since this transformation is reconstructive, involving breakage and rearrangement of chemical bonds in order to form octahedral Al – O bonds. Fig. 1 – Differential thermal analysis / thermogravimetry of seeded precursor to 1300 oC. Fig. 2 shows X-ray diffraction of calcined powders. It can be seen the evolution from transition phases into α-phase with increase of calcination temperature. Non-seeded powders (Fig. 2 (a)) showed traces of α-phase from 1050oC, while seeded powders (Fig. 2 (b)) formed α-alumina at temperatures as low as 900oC. This results show that alumina seeds played an important role on the reduction of phase transformation temperatures, although at all calcination temperatures there can be found traces of transition aluminas (mostly γ-alumina). Powders calcinated at 1150 and 1200oC also showed peaks of β-alumina (Na2O.11Al2O3), possibly with Na+ cations partially substituted by K+ cations. This phase has variations on stoichiometry in function of temperature and crystallizes only at temperatures above 1100 oC. The formation of this phase was unexpected, however it was possible due to the residual sodium and potassium present after calcination of egg white. EDS results showed presence of small amounts of these elements in the calcinated powders, confirming DRX results. (a) (b) Fig. 2 – X-ray diffractometry of calcined alumina powders for 2 hours. (a) non-seeded (b) seeded. Conclusions Changes on the surface of titanium by plasma nitriding can promote changes in the properties of its surface. These properties are related to the biological response of the surface. In this work, the evaluation of the biologic response relative to the surface of the new biomaterial showed that the behavior of osteoblasts, was influenced by the roughness of the surface wettability and topography of titanium subjected to this treatment. The roughness values obtained were significantly different between the smooth samples and treated samples and in competition between the two surfaces, the cell not only had preference for the treated part, but this was more intense than in the case of exposure of the surface to surface completely treated. References [1] Dhara, S., 2005. Synthesis of nanocrystalline alumina using egg white. J. Am. Cer. Soc., 88, 2003-2004. [2] Al-Angari, Y. M., 2011. Magnetic properties of La-substituted NiFe2O4 via egg-white precursor route. J. Mag. Mag. Mat., 323, 1835-1839. [3] Kumagai, M.; Messing, G. L., 1984. Enhanced densification of boehmite sol-gels by αalumina seeding. J. Am. Cer. Soc., 67, 230-231. [4] Shiau, F. S.; Fang, T. T., 1999. Low temperature synthesis of α-alumina using citrate process with α-alumina seeding. Mat. Chem. Phys., 60, 91-94. Acknowledgement: CAPES, Fundação Araucária, CNPq.