Radical prostatectomy for localized prostate cancer – UpToDate
Authors:
Eric A Klein, MD
Section Editors
Nicholas Vogelzang, MD
W Robert Lee, MD, MS, MEd
Jerome P Richie, MD, FACS
Deputy Editor
Michael E Ross, MD
Last literature review version 18.2: May 2010 |
This topic last updated: April 1, 2010
INTRODUCTION — Prostate cancer is the sixth most common cancer in the
world, and it is the most common non-skin malignancy in the United States
where it represents about 30 percent of all cancers diagnosed in men each year.
Widespread screening with prostate specific antigen (PSA) has led to increased
detection of prostate cancer when the tumor is localized and therefore potentially
curable. (See "Screening for prostate cancer", section on 'Evidence from
observational studies'.)
Standard management options for men with clinically localized prostate cancer
include radical prostatectomy (RP), radiation therapy (RT), including external
beam and/or brachytherapy, and active surveillance (watchful waiting).
RP is an effective option for localized prostate cancer, based upon long-term
cancer control rates, perioperative morbidity and mortality rates, and the
associated profiled of long-term adverse effects. For men choosing RP, surgical
options include both the retropubic and perineal approaches, as well as minimally
invasive (robotic or laparoscopic) surgery.
The uncertainty about optimal therapy for early prostate cancer is reflected in the
lack of definitive recommendations from both the American Urological
Association (AUA) task force on treatment for early stage prostate cancer and
the National Comprehensive Cancer Network (NCCN) guidelines [1,2]. The 2007
AUA guidelines concluded that the available data are insufficient to recommend
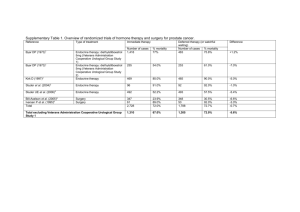
any one form of treatment over another for any risk category of disease (table
1) [1].
The surgical approaches to the treatment of clinically localized prostate cancer
will be reviewed here. The issues and data that compare surgery with other
treatment modalities are addressed elsewhere. (See "Overview of treatment for
clinically localized prostate cancer".)
PATIENT SELECTION — PSA-based screening has increased the frequency
with which patients are diagnosed with localized disease so that most newly
diagnosed men are candidates for definitive therapy. The best candidates for RP
are those with a life expectancy of 10 years or more and no or minor
comorbidities.
The probability of long-term disease control following RP is highest in patients
with cancers confined to the prostate gland (clinical stage T1 or T2). Additional
prognostic information is derived from the pretreatment serum PSA level and the
biopsy Gleason score, and this information has been combined into the anatomic
stage prognostic groups of the 2010 TNM staging system (table 2 and table 3). In
the Partin model, this information has been used to construct tables that estimate
the likelihood of organ-confined cancer with various combinations of these values
(table 4) [3]. (See "Early stage prostate cancer: Predicting the pathologic extent
of disease and clinical outcome", section on 'Predictive tools'.)
RP is also an accepted treatment option for men with locally advanced (T3) and
high-risk prostate cancer, many of whom will have lymph node involvement. The
role of surgery in the management of patients with T3 prostate cancer is
discussed elsewhere. (See "Clinical stage T3 prostate cancer", section on
'Radical prostatectomy'.)
Commonly encountered comorbidities such as diabetes mellitus, hypertension, or
coronary heart disease (CHD) are not contraindications to surgery in otherwise
healthy patients with well-controlled blood glucose and blood pressure and
normal exercise capacity. Patients with known CHD and those deemed to be at
increased risk should undergo routine preoperative cardiac risk assessment.
(See "Estimation of cardiac risk prior to noncardiac surgery".)
RETROPUBIC RP: PROCEDURE
Preoperative preparation — Preoperative counseling that includes the spouse or
partner is essential to address the practical and emotional issues surrounding
RP. Presurgical psychosocial interventions including a focus on stress
management may be useful in improving quality of life following surgery [4].
One of the most important goals is to set reasonable expectations for the shortterm and long-term effects of surgery on activity level, continence, and potency.
Special emphasis is placed on the type of anesthesia to be used, whether or not
lymphadenectomy will be performed, and whether or not a nerve-sparing
procedure is contemplated, as well as the anticipated length of hospital stay.
Many men choose to donate autologous blood preoperatively. However, in our
experience, the non-autologous transfusion rate is identical (about 5 percent)
whether or not autologous blood is available. In addition, many of these units will
be unused and therefore discarded [5]. Refusal of blood storage or transfusion
on religious grounds is not a contraindication to RP. (See "Preoperative
autologous blood donation".)
The diet is restricted to clear liquids on the day prior to surgery, and a Fleet
enema is administered the evening before or morning of surgery. Patients are
admitted directly to the operating room (OR) on the day of surgery. A secondgeneration cephalosporin is routinely administered intravenously on call to the
operating room and for two doses postoperatively.
Anesthesia considerations — Retropubic RP can be safely performed under
general, epidural, or spinal anesthesia.
Epidural anesthesia is our preferred technique for all patients undergoing
retropubic RP. Epidural anesthesia avoids the need for ventilatory support and
virtually eliminates pulmonary complications. In our experience, epidural
anesthesia alone is associated with better postoperative analgesia (as assessed
by patient self-report using validated instruments), less sedation, lower opiate
use, fewer transfusions, and less expense compared to epidural plus general
anesthesia (table 5).
Low thoracic epidural catheters are placed preoperatively and dosed with 0.1
percent bupivacaine and morphine sulfate (0.05 mg/mL) upon arrival in the
operating room. This approach promotes early return of intestinal function [6].
Analgesia is maintained intraoperatively and postoperatively with
morphine sulfate or fentanyl, and anxiolytics are administered parenterally as
needed. This regimen has been associated with a high degree of patient
acceptance and few complications, with approximately 5 percent requiring
conversion to general anesthesia.
Surgical technique — For a retropubic RP, the patient is placed in the supine
position with slight hyperextension at the iliac crest. A Foley catheter is placed
prior to incision. A midline incision is carried from the umbilicus to the top of the
pubis, usually 10 to 15 cm in length depending upon individual patient anatomy.
The space of Retzius is developed bluntly and the bladder is mobilized off the
pelvic sidewall bilaterally. The peritoneum is mobilized superiorly, exposing the
psoas muscles bilaterally.
Dissection and removal of the prostate — The apical dissection is the most
challenging part of a retropubic RP because of the close anatomic relationship
with the dorsal vein complex, neurovascular bundles, and the distal sphincter. A
meticulous apical dissection can limit blood loss. Modifications in the technique of
apical dissection significantly affect the approach to nerve sparing and can
improve the return of urinary control and potency.
After incision of the endopelvic fascia, the puboprostatic ligaments are routinely
divided along the posterior aspect of the pubis to obtain better exposure of the
dorsal vein complex. The puboprostatic ligaments support the proximal
pendulous urethra and attach the membranous urethra and the striated sphincter
to the underside of the pubis [7]. This urethral suspensory mechanism may play
an important role in maintaining effective distal sphincter function. In at least two
reports, preservation of the puboprostatic ligaments was associated with earlier
return of urinary control, less blood loss, and did not compromise margin status
[8,9].
A variety of methods have been proposed for division and control of the dorsal
vein [10-12]. Control of the dorsal vein complex is critical for achieving
hemostasis and for exposure of the prostatic apex. Preservation of the striated
urethral sphincter, which lies just underneath the dorsal vein and envelops the
urethra and prostatic apex, is essential for the return of continence.
We use a modified technique of apical dissection, which results in a typical blood
loss of 100 to 300 mL from the dorsal vein prior to suture ligation, urethral
exposure and preservation of urethral length, and excellent visualization of the
neurovascular bundles at the apex prior to their dissection [13]. This technique
involves initial incision of the endopelvic and lateral pelvic fascia, sparing of the
puboprostatic ligaments, mobilization of the neurovascular bundles away from
the prostate, and mobilization of the prostate off the anterior rectal surface prior
to urethral transection [14].
The remainder of the procedure is similar to other published techniques and
includes division of the urethra, placement of the vesicourethral anastomotic
sutures, division of the bladder neck, dissection of the seminal vesicles, and
completion of the vesicourethral anastomosis. A detailed description of our
technique has been published elsewhere [13]. Others have used similar surgical
modifications and reported comparable results [15-17].
Following completion of the vesicourethral anastomosis, closed suction drains
are placed through separate incisions through the body of the rectus muscle and
left in the obturator fossa. Only a single drain is used for patients in whom a
pelvic lymphadenectomy is not performed. The incision is closed in a single layer
with running nonabsorbable suture and the skin is approximated with clips.
Average operative time (skin incision to skin closure) for RP without and with
pelvic lymphadenectomy is 110 and 125 minutes, respectively.
The average blood loss with a retropubic RP is 800 to 1200 mL [18-21]; massive
blood loss is rare. Because of improvements in surgical technique and reduction
in transfusion triggers, intraoperative blood transfusion is needed in 20 percent of
cases or fewer [20,22-24].
Nerve-sparing approach — Erectile function following RP depends upon
preservation of the autonomic cavernous nerves, located within the
neurovascular bundles, immediately posterolateral to the prostate capsule, within
the periprostatic fascia [25]. In order to maintain potency, every effort should be
made to preserve the bilateral neurovascular bundles as long as cancer control
rates are not adversely affected. (See 'Impotence' below.)
Oncologic outcomes are not compromised by the use of the nerve-sparing
approach if the tumor is confined to the prostate [26,27]. The decision of whether
or not to perform a nerve-sparing approach is made during surgery based upon
visual inspection and palpation of the gland and its relationship to the nerve
bundle.
Various preoperative parameters (eg, clinical stage, Gleason score, preoperative
serum PSA) may help predict whether a nerve-sparing approach is likely to be
feasible [28]. Dynamic endorectal coil MRI may also be useful for preoperative
identification of neurovascular bundle involvement [29,30]. (See "Clinical stage
T3 prostate cancer".)
Some series suggest that nerve-sparing RP can be performed safely in men with
pathologic extraprostatic extension as long as the neurovascular bundles are not
involved. The posterolateral margin is one of the least common sites of
involvement in men with positive margins, being positive in only 10 to 17 percent
of cases [31-33]. If there is a clinical suspicion of extraprostatic extension,
intraoperative frozen section analysis of the posterolateral margin can be used to
predict tumor involvement of the neurovascular bundle and the advisability of a
nerve-sparing approach [34]. If the posterolateral margin is histologically
negative, the neurovascular bundles are usually uninvolved.
For men who are unsuitable for a nerve-sparing procedure, sural or
genitofemoral nerve grafts may restore potency in some patients who undergo
resection of both neurovascular nerves [35-37].
Pelvic lymph node dissection — The role and extent of pelvic lymph node
dissection (PLND) in men undergoing RP for prostate cancer is controversial.
PLND is the standard approach for assessing regional lymph node status, which
can provide important information about prognosis. Whether or not PLND offers a
therapeutic benefit is unclear, and its prognostic value must be balanced against
potential complications.
The techniques for regional lymph node assessment and the management of
patients with positive nodes are discussed separately. (See "Evaluation of
regional lymph nodes in men with prostate cancer" and "Management of prostate
cancer patients with positive regional lymph nodes".)
Postoperative care — Patients are ambulated on the evening of or the morning
following retropubic RP. A clear liquid diet is begun on postoperative day 1 and
advanced as tolerated. Analgesia is maintained with continuous and on-demand
morphine sulfate plus bupivacaine via epidural catheter for 24 hours, followed by
oral ketorolac and ibuprofen as needed. The drains are removed after 48 hours
unless there is clinical suspicion of a urine leak. Most patients are discharged
after two nights of hospitalization, returning five to seven days later for the
removal of incisional staples and the Foley catheter.
This accelerated postoperative regimen is well tolerated by patients, and reduces
the cost associated with retropubic RP without compromising the quality of care
based upon the frequency of acute complications, hospital readmissions, or
mortality [38].
RETROPUBIC RP: OUTCOME — Several institutions have published long-term
efficacy data for patients with prostate cancer treated with retropubic RP [39-45].
The most widely reported clinical end point is the incidence of a detectable serum
PSA level following surgery, the absence of which is expressed as the
biochemical relapse-free survival rate (bRFS). Although results vary,
approximately 70 percent of men undergoing RP for clinically localized disease
have control of disease for at least 10 years based upon this criterion.
Significance of biochemical failure — PSA-defined biochemical recurrence is a
widely accepted endpoint in analyzing outcomes following RP. Although various
definitions of biochemical recurrence have been proposed, the most widely
accepted criterion for patients who have undergone RP is that of the American
Urological Association (AUA) [46]. According to AUA guidelines, a biochemical
recurrence is defined as a serum PSA ≥0.2 ng/mL, which is confirmed by a
second determination with a PSA ≥0.2 ng/dL.
Biochemical failure after definitive local treatment antedates metastatic
progression and prostate cancer-specific mortality by an average of 7 and 15
years, respectively [47-49].
The complex relationship between biochemical recurrence and long-term
outcome is illustrated by the following observations:
In a consecutive series of 1132 men with clinically localized disease, 19
percent had a biochemical relapse, one-fourth of whom developed metastases at
10 years [47]. However, the 10-year survival rate in those with a PSA recurrence
was similar to that in men without biochemical failure (88 and 93 percent,
respectively).
A second report of 1197 men observed a biochemical relapse after RP in
15 percent, of whom 34 percent developed metastatic disease at a median of
eight years [48]. The median time to death after the development of metastases
was five years. Men with a rising serum PSA within two years were less likely to
be free of clinically evident metastases at seven years compared with those who
failed after two years, both for Gleason score 5-7 disease (47 versus 77 percent),
and Gleason score 8-10 disease (21 versus 47 percent).
In a third series of 2809 men undergoing RP for ≤T2 disease (table 2),
biochemical failure developed in 31 percent, at an average of 2.9 years
posttreatment [49]. Among men with a biochemical failure, 91 percent remained
free of systemic progression 10 years after a rising PSA was first detected.
Effect of pathologic features — The prognosis following retropubic RP depends
upon pathologic characteristics of the tumor, such as seminal vesicle or nodal
involvement, margin status, and the presence of extraprostatic extension
[39,41,42,44,50].
The importance of these factors is illustrated by the outcomes in our series of
906 men undergoing retropubic RP at the Cleveland Clinic for clinically localized
disease [44]. In this cohort, 43 percent had extraprostatic extension, 56 percent
had Gleason score ≥7 disease, 23 percent had positive margins, 9 percent had
seminal vesicle invasion, and 2 percent had nodal metastases [44].
At an average follow-up of 44 months, the five- and eight-year cancer-specific
survival rates for the entire cohort were 97 and 95 percent, and the biochemical
relapse-free survival (bRFS) rates were 81 and 76 percent, respectively.
Results based upon the presence or absence of various pathologic features
included the following:
Men with organ-confined disease (no extraprostatic extension and
negative surgical margins) had a 100 percent cancer-specific survival and a 92
percent bRFS rate at both five and eight years.
In men with extraprostatic extension who had negative or positive
margins, the eight-year bRFS was 77 and 50 percent, respectively.
Men with seminal vesicle invasion or lymph node metastases had the
worst outcomes (34 and 0 percent bRFS at eight years, respectively).
Combining all pathologic parameters, six prognostic groups could be identified to
define risk groups for biochemical failure following RP (figure 1) [44]. These
observations permit the identification of men at higher risk for treatment failure
who might be candidates for immediate hormone ablation or other therapies.
(See "Initial hormone therapy for metastatic prostate cancer".)
Cause-specific mortality — Although most series utilize biochemical relapse-free
survival (bRFS) as the clinical end point, disease-specific mortality provides
important insights into outcomes following radical prostatectomy.
The relationship between disease-specific mortality and various clinical and
pathologic variables was evaluated in a multi-institutional cohort series of 12,677
men who underwent RP for clinically localized disease between 1987 and 2005
[51]. The 15-year disease-specific and overall mortality rates were 12 and 38
percent, respectively, for the entire cohort.
The main factors affecting cancer-specific mortality at 15 years included:
Clinical stage:
- Stage T1 — 6 percent
- Stage T2a — 7 percent
- Stage T2b — 14 percent
- Stage T2c — 12 percent
- Stage T3 — 38 percent
Pretreatment serum PSA (ng/mL):
- <4 — 4 percent
- 4 to 10 — 9 percent
- 10.1 to 20 — 11 percent
- 20.1 to 50 — 22 percent
Biopsy Gleason score:
- 2 to 6 — 6 percent
- 7 — 17 percent
- 8 to 10 - 34 percent
Because of change in pathologic criteria, Gleason scores are now consistently
higher than when these data were generated. Thus, the Gleason score results
from this study cannot be directly applied to current practice. (See "Interpretation
of prostate biopsy", section on 'Gleason score'.)
There was a statistically significant improvement in prognosis for patients
diagnosed more recently. Whether this reflects an improvement in treatment,
changes in Gleason grading, or an effect of PSA screening is uncertain.
These data have been used to generate a nomogram to assist in treatment
planning for newly diagnosed patients. (See "Early stage prostate cancer:
Predicting the pathologic extent of disease and clinical outcome", section on
'Predictive tools'.)
PSA kinetics — A short PSA doubling time or a rapid PSA velocity prior to RP
correlate with increased mortality from prostate cancer in men managed with
retropubic RP. This correlation was illustrated by a series of 1095 men, in which
a PSA velocity of 2 ng/mL per year or higher was associated with significantly
shorter time to recurrence, and higher rates of death from prostate cancer
(relative risk [RR] 12.8, 95% CI 3.7 to 43.7) relative to those with a PSA velocity
<2 ng/mL per year [52].
Surgeon experience — Long-term oncologic results are influenced by the
experience of the surgeon.
The correlation between surgical experience and long-term oncologic outcomes
was illustrated in a series of 7765 men who underwent RP by one of 72 surgeons
at four US academic medical centers between 1987 and 2003 [53]. The learning
curve was steep and did not start to plateau until a surgeon had performed 250
retropubic RPs. The predicted probability of biochemical recurrence at five years
was significantly lower when the surgeon had performed 250 prior operations
(10.7 versus 17.9 percent when the surgeon had performed 10 prior operations).
The benefit from increasing surgical experience was present for men whose
prostate cancer was at low, medium, and high risk of recurrence [54].
These observations suggest that men wanting the best chance for cure should
seek the most experienced available surgeon.
Predicting recurrence — A number of nomograms to predict long-term individual
outcomes following retropubic RP have been developed and validated in both
Caucasian and African-American men [55]. These nomograms can incorporate
demographic data such as age, Gleason score, clinical stage, and treatment
plans to help estimate the risk of recurrence or progression in a variety of clinical
scenarios.
A number of these nomograms developed by the Memorial Sloan-Kettering
group are accessible on line at www.mskcc.org/mskcc/html/10088.cfm
RETROPUBIC RP: QUALITY OF LIFE — The complications of most concern to
men who undergo retropubic RP are urinary incontinence and impotence, which
are due to operative damage to the urinary sphincter and penile nerves,
respectively. On the other hand, removal of the prostate may partially reverse or
prevent the progression of symptoms due to antecedent benign prostatic
hyperplasia, potentially improving quality of life.
Perioperative complications — Perioperative morbidity rates are generally under
10 percent. Serious complications include myocardial infarction, and
thromboembolic, infectious, and neurologic events [18-20,39,56]. Operative
mortality rates in most reported series are less than 1 percent, even in older men
[16,19,40,41,57].
The perioperative complication rate in our most recent series of 306 consecutive
retropubic RPs was 8.2 percent (table 1) [18]. Most complications were minor
and resolved without sequelae.
Patient age — Perioperative complications are more common in older men,
although this may be due in part to comorbidities. The effect of age on the
incidence of complications is illustrated by the following examples:
In a series of 1870 men managed with retropubic RP by one surgeon, the
perioperative complication rates among men in their 40s, 50s, 60s and 70s were
4, 9, 11, and 14 percent, respectively [19].
In a report of 11,010 men who underwent RP in Ontario, Canada between
1990 and 1999, comorbidity was a stronger predictor than age of almost all
categories of early complications [57]. Even when adjusted for comorbidity and
year of surgery, age was associated with a significantly increased risk of 30-day
mortality (odds ratio 2.04 per decade of age). Nevertheless, the absolute 30-day
mortality risk was low, even in men aged 70 to 79 (0.66 percent versus 0.58 and
0.19 percent in men 60 to 69 and <60 years of age, respectively).
Hospital volume — Hospitals with higher volumes of RPs tend to have shorter
lengths of hospital stay, lower perioperative morbidity and mortality rates, and a
reduced risk of serious treatment-related complications [58,59].
This relationship was illustrated by a series that used the Surveillance,
Epidemiology and End Results (SEER)-Medicare linked database, in which
perioperative morbidity rates were significantly different (27 versus 32 percent)
when very high volume hospitals (114 to 252 procedures per year) were
compared to low volume institutions (fewer than 33 procedures annually) [58].
Similar results were noted when very high volume surgeons were compared to
low volume surgeons. Furthermore, significant differences were also apparent
between very high and low volume institutions when the rates of long term
urinary complications (but not urinary incontinence) were compared.
Urinary incontinence
Incidence — Complete urinary incontinence is uncommon following retropubic
RP. The use of bladder neck sparing and nerve-sparing approaches has
decreased the incidence of urinary incontinence from bladder neck contracture to
1 to 2 percent [8,10,11,18,60-62]. Nevertheless, the majority of men experience
some degree of urinary incontinence after RP, particularly stress incontinence.
The incidence of incontinence depends upon the source of the data (physicianreported versus patient-reported symptoms), the definition of continence, the
time elapsed since surgery, and whether or not a nerve-sparing approach was
used.
In physician-reported series from single institutions, the reported rates of
continence (typically defined as no or minimal urinary leakage) ranges from 88 to
100 percent at 6 to 24 months following surgery [11,12,60,63,64]. In one of the
largest reports, which included 581 men who were continent prior to RP,
independent factors associated with higher rates of regaining continence were
younger age, preservation of both neurovascular bundles, and absence of an
anastomotic stricture [11]. In patients operated on after 1990, the median time to
regain continence was 1.5 months and the rate of continence at 24 months was
95 percent. The influence of nerve-sparing versus non-nerve-sparing surgery
was illustrated in a report in which the median time to recover continence
(patient-defined as the absence of any urinary leakage) was significantly shorter
in men undergoing nerve-sparing surgery (5.3 versus 10.9 months) [65].
Patient-reported data suggest that the frequency of incontinence is higher.
In the Prostate Cancer Outcomes Study, results from 1291 men aged 39 to 79
who underwent retropubic RP for localized prostate cancer over a one year
period (1994 to 1995) were analyzed [66]. At 24 months after surgery, 1.6
percent reported no urinary control (compared with 0.7 percent at baseline prior
to surgery), while 7 and 42 percent reported frequent and occasional leakage,
respectively (compared with 2 and 9 percent at baseline) (table 6). The incidence
of incontinence increased with age (14 percent in men ages 75 to 79 compared
with 0.7 to 4 percent in younger age groups).
Time course — Urinary incontinence typically improves with time following
retropubic RP [62,67-70]. Most men achieve continence within three to six
months, although it can take up to 18 to 24 months. In our experience,
continence without the need for absorptive pads is achieved by 90 percent of
men at a median of six weeks, with stress incontinence requiring one or two pads
per day in 10 percent. Less than 1 percent require surgical placement of an
artificial sphincter.
In a series of 25,651 men in the Medicare population who underwent RP in 1991,
urinary incontinence was reported in 22 percent after surgery, but only 8 percent
continued to carry that diagnosis ≥12 months later [67]. Centers in which
retropubic RP is performed in large numbers by one or only a few surgeons
generally report better outcomes than those seen in the Medicare-based survey.
Management — Options available for the management of urinary incontinence
include conservative maneuvers, periurethral injections of collagen, the urethral
sling procedure, and the artificial urinary sphincter.
Conservative management options (eg, pelvic floor muscle training, biofeedback,
electrical stimulation using a rectal electrode, transcutaneous electrical nerve
stimulation) are often used in the first months following RP in an effort to control
symptoms while sphincter function is returning. Data supporting these
approaches are limited [71-73].
One randomized trial has observed a significant decrease in the rates of
incontinence with pelvic floor reeducation (PFR) during the first months following
RP in men who were incontinent 15 days after RP [72]. Compared to a placebo
procedure, the use of PFR was associated with a significant decrease in the rate
of incontinence at three (10 versus 44 percent) and 12 months (4 versus 17
percent).
Periurethral injection of collagen offers a minimally invasive approach for the
treatment of men with incontinence following RP, but success rates with this
approach are limited [74-77]. As an example, in one series of men with
postprostatectomy incontinence, 8 of 41 (20 percent) treated with collagen
injections were at least socially continent, requiring one pad daily or less [75].
More invasive options for patients with severe incontinence include the insertion
of an artificial urinary sphincter and the urethral sling procedure:
Artificial urinary sphincter — The artificial urinary sphincter (AUS) is a
surgically implanted device that circumferentially occludes the urethra, thereby
preventing urinary leakage [74]. The device consists of a reservoir that is
connected to a pump inserted in the scrotum, which the patient can activate to
temporarily relieve the pressure, thereby permitting voiding. Pressure on the
urethra can cause ischemia, which may necessitate revision or removal of the
AUS.
The most extensive experience reported with the AUS comes from the Mayo
Clinic [78]. At an average follow-up of 6.5 years, the rate of continence following
placement of an AUS was 88 percent in 323 patients with severe incontinence
(70 percent due to RP). Revisions to the AUS were needed in about one-third of
cases.
Urethral sling procedure — The urethral sling procedure involves the
insertion of a noncircumferential support that compresses the urethra [74]. The
amount of pressure on the urethra is adjusted at the time of surgery such that the
detrusor activity of the bladder can overcome this obstruction to permit voiding.
Several series have evaluated this procedure in men with severe incontinence
[79-81]. One report, for example, included 48 men with severe incontinence
following RP at a median follow-up of 48 months [79]. The outcome was
excellent (no use of pads) in 31 (65 percent) and another seven patients (15
percent) had substantial improvement.
The AUS is preferred in patients who have had a prior procedure for urinary
incontinence, patients who have had prior RT, and those who have poor bladder
detrusor activity [74]. A sling procedure is preferable in men who are unable to
manipulate the AUS reservoir.
Retropubic RP versus RT — The relative frequency with which incontinence
occurs following retropubic RP compared to radiation therapy is discussed
separately. (See "Overview of treatment for clinically localized prostate cancer",
section on 'Complications of EBRT'.)
Bladder neck contracture — Urethral strictures following radical prostatectomy
may be due to bladder neck contracture or narrowing of the urethral at more
distal sites. Urethral stricture may be manifested by symptoms of decreased
urinary stream or overflow incontinence.
In a series of 3310 men from the CaPSURE database managed with radical
prostatectomy, urethral stricture was diagnosed in 8 percent [82]. In contrast,
urethral strictures were diagnosed in approximately 1 percent of patients
managed with watchful waiting. In another series of 1289 men who underwent
radical prostatectomy between 1998 and 2004, 138 (11 percent) developed a
bladder neck contracture [83]. The incidence was significantly higher among men
who had urinary retention within seven days after catheter removal.
Most contractures can be easily managed with simple dilation. Dense
strictures may require endoscopic incision [84].
Impotence — The frequency of impotence following retropubic RP depends upon
patient age, preoperative sexual function, and whether or not nerve-sparing
surgery was performed. (See 'Nerve-sparing approach' above.)
Incidence — Potency can be preserved in many men with normal preoperative
erectile function who undergo bilateral nerve-sparing RP [17,18,38,62,63,85].
Using phosphodiesterase inhibitors such as sildenafil, potency rates as high as
76 to 86 percent have been reported from individual surgeons and centers
performing nerve-sparing surgery on carefully selected men [63,85].
In contrast, the potency rate was substantially lower in an American College of
Surgeons survey of hospital tumor registry data of multiple hospitals with varying
expertise [86]. Only 28 to 30 percent of men undergoing RP between 1990 and
1993 maintained erectile function adequate for intercourse after surgery.
Patient estimates of the frequency of impotence may differ substantially from
physician-reported single-center data. For example, in the Prostate Cancer
Outcomes Study, 42 percent of men undergoing RP reported that their sexual
performance was a moderate to large problem at 24 months (compared with 18
percent at baseline), and 60 percent were not able to have erections firm enough
for sexual intercourse (compared with 16 percent at baseline) (table 7) [66].
The return of potency is gradual. In a report of 647 previously potent men
undergoing retropubic RP at a tertiary referral center between July 1998 and July
2003, the probabilities of recovering potency at the end of postoperative years 1
and 2 were 37 and 62 percent, respectively [87].
The likelihood of potency following RP is age related. In one series, the potency
rate after surgery was 86 percent in men in their 40s, and 80, 60, and 42 percent
for men in their 50s, 60s, and 70s, respectively [63].
Retropubic RP versus radiation — The relative frequency with which impotence
occurs following RP compared to RT as primary therapy for localized prostate
cancer is discussed separately. (See "Overview of treatment for clinically
localized prostate cancer", section on 'Complications of EBRT'.)
Management — Penile sensation and the ability to have an orgasm are
preserved even if the erectile nerves are removed during RP, leaving several
options for treatment of erectile dysfunction (ED) in these men [88]. The
treatment approaches for ED are discussed separately. (See "Treatment of male
sexual dysfunction" and "Surgical treatment of erectile dysfunction".)
The success of medical treatments for men with ED who are long-term survivors
of prostate cancer is limited. Men prefer noninvasive treatments although
invasive treatments (intracavernosal injection therapy, penile implants,
prostheses) are more effective [89]. Sexual counseling should be recommended
for men and their partners, as it may increase the use of and satisfaction with
medical therapies.
Phosphodiesterase inhibitors such as sildenafil are most helpful in men who have
undergone a nerve-sparing procedure [85,89-92]. As an example, in one study of
91 men presenting with ED following RP, the response rates to sildenafil in men
who had undergone bilateral nerve-sparing, unilateral nerve-sparing, and a nonnerve sparing approach were 72, 50, and 15 percent, respectively [92].
The response to sildenafil increases with time following RP. In a study in which
95 percent of men had undergone nerve-sparing procedures, 60 percent
reported benefit from sildenafil at 18 to 24 months after surgery, significantly
higher than the 29 percent who reported benefit in the first six months after
surgery [93].
Nightly treatment with a phosphodiesterase inhibitor beginning after radical
prostatectomy has been advocated to improve erectile function long term [94],
However, on-demand treatment appears to be equally effective. In a randomized,
double-blind trial, 628 men were randomly assigned to nightly vardenafil, ondemand vardenafil, or placebo for nine months [95]. After a two-month washout
period, there were no differences in sexual performance between the two
vardenafil regimens, and on-demand vardenafil was better than placebo
throughout the trial.
Inguinal hernia — The incidence of inguinal hernias appears to be increased
following retropubic RP, due both to the detection of preexisting hernias that
were not diagnosed preoperatively as well as alterations of inguinal anatomy
during surgery [96,97].
This was illustrated by a consecutive series of 1130 patients, in whom 13 percent
had a preexisting hernia and 8 percent developed a new hernia postoperatively
[96]. In contrast, the incidence of inguinal hernia appears to be much lower
following perineal RP (5 of 285 [1.8 percent] versus 32 of 311 [10.3 percent]
following retropubic RP) [98].
BPH symptoms — Men who undergo RP for localized prostate cancer frequently
have pretreatment lower urinary tract symptoms that are due to benign prostatic
hyperplasia (BPH) rather than tumor. RP may partially reverse or prevent the
progression of such symptoms.
The impact on lower urinary tract symptoms was assessed in a study of 453 men
who underwent an RP for localized prostate cancer and who completed the
American Urological Association symptom score (AUASS) at baseline, and 12
and 48 months after surgery (table 8) [99]. Overall, 36 percent of patients had
moderate or severe symptoms at baseline (AUASS ≥8), while 64 percent had no
or mild symptoms (AUASS ≤7).
Among men with moderate to severe symptoms at baseline, there was a
clinically and statistically significant decrease in symptoms at 12 months, which
was maintained at 48 months (AUASS -5.0 versus baseline and -6.3 versus
baseline at 12 and 48 months, respectively). The improvement in symptoms
occurred despite the discontinuation of medical therapy for symptomatic BPH in
all patients after RP.
In men with mild symptoms, there was an initial slight worsening of
symptoms in the first 12 months without further subsequent deterioration
(AUASS +2.0 versus baseline and +1.7 versus baseline at 12 and 48 months,
respectively). Although these increases from baseline were statistically
significant, they were not clinically relevant.
These results were derived from a nonrandomized series. Although the natural
history of BPH can be variable, the symptoms of BPH in some men appear to
improve following surgery. A prospective study found that the benefit of RP in
men with obstructive or irritative urinary symptoms was greatest in those with
large prostate size [62]. The potential impact of RP on such symptoms should be
considered when choosing definitive treatment of localized prostate cancer [99].
(See "Clinical manifestations and diagnosis of benign prostatic hyperplasia",
section on 'Natural history'.)
MINIMALLY INVASIVE RP — Minimally invasive RP (laparoscopic or robotic)
has been developed as an alternative to retropubic RP [100-107]. This approach
uses a smaller incision and provides magnification of the surgical field for the
operator. Postulated advantages of a minimally invasive RP include fewer
complications and quicker recovery.
There are no randomized trials that compare minimally invasive RP to retropubic
RP for prostate cancer. The most extensive data come from an analysis of 8837
men identified from the Surveillance, Epidemiology and End Results (SEER)
Medicare database who underwent RP [100].
Key results from this study included the following:
Utilization of minimally invasive RP increased from 9 percent of cases in
2003 to 43 percent in 2006-2007.
Compared to retropubic RP, minimally invasive RP was significantly
associated with shorter hospital stays (2.0 versus 3.0 days), less frequent blood
transfusions (2.7 versus 20.8 percent), fewer respiratory complications (4.3
versus 6.6 percent), and fewer anastomotic strictures (5.8 versus 14.0 percent).
Compared to retropubic RP, minimally invasive RP was associated with a
significantly increased risk of genitourinary complications (4.7 versus 2.1
percent), incontinence (15.9 versus 12.2 per 100 person-years), and erectile
dysfunction (26.8 versus 19.2 per 100 person-years).
There are conflicting data about whether or not there is a higher rate of positive
surgical margins following minimally invasive RP compared to retropubic RP:
In a series of 400 patients from Vanderbilt, lower rates of positive margins
were observed in patients treated with minimally invasive RP compared to those
undergoing retropubic RP (9 versus 24 percent for pathologic T2 disease and 50
versus 60 percent for pathologic T3 disease) [101]. However, the better results
with minimally invasive RP may have been due to more favorable patient
characteristics in this group (lower Gleason scores, lower PSAs, and lower
clinical stage). In addition, the observed rates of margin positivity with minimally
invasive RP were much higher than those reported by other groups with
retropubic RP [108].
Indirect evidence supporting a higher incidence of margin positivity comes
from an analysis of 2702 men in a Medicare database, which found that those
treated with a minimally invasive RP were significantly more likely to require
salvage RT or injectable hormonal treatment within six months after their RP (28
versus 9 percent after open RP) [105].
There is a significant learning curve associated with laparoscopic and robotic RP
[102,109,110]. As an example, the rates of complications and the need for
salvage treatment in a Medicare database analysis were substantially higher for
both minimally invasive and open RPs compared to those reported from highvolume institutions [102].
PERINEAL RP — A perineal approach for RP is a reasonable alternative for
appropriately selected men with localized prostate cancer. This approach can be
combined with preservation of the neurovascular bundles, but is not suitable for
patients in whom pelvic lymph node dissection is required.
Perineal versus retropubic RP — Perineal RP has been compared to retropubic
RP in one randomized trial and several retrospective observational studies [111113]. These reports indicate that perineal RP has similar oncologic outcomes in
carefully selected patients with localized prostate cancer, although there are
differences in the frequency of side effects with the two procedures.
The two approaches were compared in an Italian trial, in which 200 men with
clinical stage T1 or T2 prostate cancer and a prostate gland weighing less than
80 g were randomly assigned to either a perineal RP or a retropubic RP [111].
Surgery for all patients was performed by a single surgeon who was trained in
both techniques.
The following findings were noted:
At a mean follow-up of 60 months, there was no difference in biochemical
relapse-free survival, with 12 and 11 percent having a serum PSA >0.2 ng/mL
with perineal and retropubic RPs, respectively. There was also no difference in
the incidence of positive surgical margins (14 and 15 percent, respectively).
The incidence and timing of return of urinary continence was the same
with both surgical approaches (74 versus 76 and 96 versus 95 percent, at 6 and
24 months, respectively).
Perineal RP was associated with significantly less blood loss, a shorter
hospital stay, and a shorter duration of catheterization compared to retropubic
RP (100 versus 400 mL, 8 versus 13 days, and 7 versus 12 days, respectively).
In contrast to these benefits, recovery of potency was significantly less
common with perineal RP (potency 30 versus 45 and 42 versus 60 percent, at 6
and 24 months, respectively).
In reports from observational series, rectal injury and fecal incontinence have
also been reported following perineal RP [112,113].
Summary — In men with localized (T1-2) prostate cancer and a relatively small
prostate gland, perineal RP is associated with less blood loss than open
retropubic RP and is associated with a similar frequency of positive margins and
biochemical relapse. However, recovery of potency is delayed and less frequent
with the perineal approach, even with a nerve-sparing procedure. There also is a
small, but increased risk of rectal injury and fecal incontinence. There are no data
comparing perineal RP with laparoscopic RP, which can also decrease blood
loss and shorten the duration of hospital stay.
Perineal RP is a reasonable option for men with low-risk primary tumors and
relatively small prostates (eg, Gleason score <6, serum PSA <10 ng/mL, and a
prostate gland <80 g). In these men, the likelihood of lymph node disease is
under 5 percent, making pelvic lymph node dissection unnecessary [114,115].
Perineal RP may be particularly useful in men who have had prior pelvic surgery
and in those for whom minimizing short-term toxicity is a priority.
ADJUVANT AND NEOADJUVANT THERAPY
Neoadjuvant ADT — Men with locally advanced prostate cancer have worse
outcomes after RP than do those with organ-confined disease (table 9). Although
neoadjuvant androgen deprivation therapy (ADT) decreased the rate of surgically
positive margins, no survival advantage was demonstrated with this approach.
(See "Clinical stage T3 prostate cancer".)
Several prospective trials of ADT before RP in men with organ-confined disease
have also noted a decrease in rates of margin positivity and improvements in
tumor size, serum PSA, final pathologic stage, risk of progression, and time to
progressive disease [116-120]. However, the few series with long-term follow-up
suggest no benefit in terms of overall or cancer-specific survival, or biochemical
relapse-free survival (bRFS):
In a randomized trial, 138 men with clinical stage T2b disease received
three months of leuprolide plus flutamide prior to RP while 144 were assigned to
immediate RP [118]. Although the positive margin rate was significantly lower
with neoadjuvant ADT (15 versus 48 percent), there was no difference in the fiveyear bRFS (serum PSA ≤0.4 ng/mL in 65 versus 68 percent of men undergoing
neoadjuvant ADT or surgery alone, respectively).
Similar results were noted in a European trial that randomly assigned 402
men with cT2-3 N0 prostate cancer to three months of goserelin plus flutamide
followed by RP or immediate RP [119] and a Canadian trial that randomly
assigned 213 men to three months of neoadjuvant cyproterone followed by RP or
RP alone [120].
Neoadjuvant chemotherapy with or without ADT — Newer approaches to locally
advanced prostate cancer consist of neoadjuvant or induction chemotherapy or
combined chemotherapy plus ADT. While chemotherapy was previously
considered to be relatively ineffective in prostate cancer, drugs such as
docetaxel produce higher rates of both objective and biochemical response, and
prolonged survival when compared to mitoxantrone. (See "Chemotherapy in
castrate-resistant prostate cancer".)
These data have led to the design of phase II trials exploring neoadjuvant
chemotherapy or chemotherapy plus ADT prior to RP in men who have adverse
pretreatment tumor characteristics. Although the ultimate goal is improved local
control and survival, initial studies were designed to demonstrate the safety of
retropubic RP following induction therapy, and to examine treated specimens for
histologic evidence of antitumor effect.
At least six trials have been reported; they included men without overt evidence
of metastases who were at high risk for extraprostatic extension and subsequent
biochemical failure by having the following characteristics [121-127]:
Pretreatment PSA >15 to 20 ng/mL OR
Clinical stage T2b, T2c, or T3 (table 2) OR
Biopsy Gleason score ≥8
All of these trials demonstrated that RP is feasible with acceptable surgical and
postoperative morbidity following neoadjuvant therapy. However, most of the
drug regimens explored (estramustine/etoposide, weekly docetaxel with or
without mitoxantrone, or combined ADT with alternating cycles of ketoconazole,
doxorubicin, vinblastine and etoposide) had no significant antitumor effect as
assessed by histologic changes in the RP specimens or a reduction in the
incidence of adverse pathologic features. A single preliminary report of a phase II
trial of weekly docetaxel plus ADT noted two complete pathologic responses
among 64 men (3 percent) [127].
Adjuvant antiandrogen monotherapy — Thus, the available evidence does not
support the use of antiandrogen monotherapy after resection of localized
prostate cancer.
Adjuvant antiandrogen monotherapy was extensively evaluated in the Early
Prostate Cancer program. In these trials, 8113 men with localized (T1, T2) or
locally advanced (T3, T4) nonmetastatic prostate cancer were randomly
assigned to bicalutamide or placebo in addition to standard care (watchful
waiting, RP, or RT) [128]. At a median follow-up of 10 years, patients with locally
advanced disease had an improvement in progression-free but not overall
survival. In men with localized (T1, T2) disease, there was no statistically
significant improvement in either progression-free or overall survival. (See
"Pathologic stage T3 and margin positive prostate cancer", section on 'Adjuvant
hormone therapy without RT'.)
Adjuvant chemotherapy — The activity of docetaxel and other cytotoxic
chemotherapy agents in patients with advanced disease has led to the evaluation
of adjuvant chemotherapy in men with resected prostate cancer. The role of
adjuvant chemotherapy following definitive local therapy of prostate cancer is
discussed separately. (See "Clinical stage T3 prostate cancer", section on
'Adjuvant therapy'.)
SUMMARY AND RECOMMENDATIONS
In patients with clinically localized prostate cancer, the preferred approach
for definitive therapy (ie, radical prostatectomy [RP] or radiation therapy [RT]) is
largely a matter of patient preference. Taken together, the available data suggest
that surgery and RT offer fairly equivalent survival outcomes, at least for the first
10 years after therapy. (See "Overview of treatment for clinically localized
prostate cancer".)
For patients who choose surgery, we suggest a retropubic RP (Grade 2C).
(See 'Patient selection' above and 'Retropubic RP: procedure' above.)
Minimally invasive RP is an alternative in situations where there is
adequate expertise with this approach. This approach has been associated with
fewer short-term complications, transfusions, and shorter hospital stays.
However, there are no comparative trials, and long term equivalence on
oncologic results has not been demonstrated. (See 'Minimally invasive
RP' above.)
Perineal RP is an alternative to retropubic RP for men with clinical stage
T1-2 disease, a relatively small prostate gland, and no indication for a lymph
node dissection. This approach may be particularly useful in men who have had
prior pelvic surgery and in those for whom minimizing short term toxicity is a high
priority. (See 'Perineal RP' above.)
We recommend that a nerve-sparing approach be incorporated into the
RP when there is no evidence of tumor involving the neurovascular bundle
(Grade 1B). This approach is associated with faster return of urinary continence
and a lower frequency of impotence in men who were potent prior to surgery.
(See 'Nerve-sparing approach' above.)
For patients with intermediate- or high-risk localized prostate cancer (ie,
PSA ≥10 ng/mL, Gleason score ≥7, or clinical stage T2b or higher) (table 2 and
table 1) who will be managed with RP, we suggest performing a pelvic lymph
node dissection prior to or in conjunction with the RP to assess the regional
lymphatics (Grade 2C). (See 'Pelvic lymph node dissection' above and
"Evaluation of regional lymph nodes in men with prostate cancer".)
Use of UpToDate is subject to the Subscription and License Agreement.
REFERENCES
1.
Thompson, I, Thrasher, JB, Aus, G, et al. Guideline for the management
of clinically localized prostate cancer: 2007 update. J Urol 2007; 177:2106.
2.
National Comprehensive Cancer Network (NCCN) guidelines are available
online at www.nccn.org.
3.
Partin, AW, Mangold, LA, Lamm, DM, et al. Contemporary update of
prostate cancer staging nomograms (Partin Tables) for the new millennium.
Urology 2001; 58:843.
4.
Parker, PA, Pettaway, CA, Babaian, RJ, et al. The effects of a presurgical
stress management intervention for men with prostate cancer undergoing radical
prostatectomy. J Clin Oncol 2009; 27:3169.
5.
O'Hara, JF Jr, Sprung, J, Klein, EA, et al. Use of preoperative autologous
blood donation in patients undergoing radical retropubic prostatectomy. Urology
1999; 54:130.
6.
Scheinin, B, Asantila, R, Orko, R. The effect of bupivacaine and morphine
on pain and bowel function after colonic surgery. Acta Anaesthesiol Scand 1987;
31:161.
7.
Steiner, MS. The puboprostatic ligament and the male urethral suspensory
mechanism: an anatomic study. Urology 1994; 44:530.
8.
Lowe, BA. Preservation of the anterior urethral ligamentous attachments
in maintaining post-prostatectomy urinary continence: a comparative study. J
Urol 1997; 158:2137.
9.
Poore, RE, McCullough, DL, Jarow, JP. Puboprostatic ligament sparing
improves urinary continence after radical retropubic prostatectomy. Urology
1998; 51:67.
10.
Walsh, PC, Quinlan, DM, Morton, RA, Steiner, MS. Radical retropubic
prostatectomy. Improved anastomosis and urinary continence. Urol Clin North
Am 1990; 17:679.
11.
Eastham, JA, Kattan, MW, Rogers, E, et al. Risk factors for urinary
incontinence after radical prostatectomy. J Urol 1996; 156:1707.
12.
Kaye, KW, Creed, KE, Wilson, GJ, et al. Urinary continence after radical
retropubic prostatectomy. Analysis and synthesis of contributing factors: a unified
concept. Br J Urol 1997; 80:444.
13.
Klein, EA, Jhaveri, F, Licht, M. Contemporary technique of radical
prostatectomy. In: Management of Prostate Cancer, Klein, EA (Ed), Humana
Press, New Jersey, 2000.
14.
Klein, EA, Kupelian, PA, Tuason, L, Levin, HS. Initial dissection of the
lateral fascia reduces the positive margin rate in radical prostatectomy. Urology
1998; 51:766.
15.
Stephenson, RA, Middleton, RG, Abbott, TM. Wide excision (nonnerve
sparing) radical retropubic prostatectomy using an initial perirectal dissection. J
Urol 1997; 157:251.
16.
Ruckle, HC, Zincke, H. Potency-sparing radical retropubic prostatectomy:
a simplified anatomical approach. J Urol 1995; 153:1875.
17.
Ohori, M, Wheeler, TM, Kattan, MW, et al. Prognostic significance of
positive surgical margins in radical prostatectomy specimens. J Urol 1995;
154:1818.
18.
Ng, CS, Klein, EA. Acute complications after radical retropubic
prostatectomy. Prostate J 2000; 2:22.
19.
Catalona, WJ, Carvalhal, GF, Mager, DE, Smith, DS. Potency, continence
and complication rates in 1,870 consecutive radical retropubic prostatectomies. J
Urol 1999; 162:433.
20.
Lepor, H, Nieder, AM, Ferrandino, MN. Intraoperative and postoperative
complications of radical retropubic prostatectomy in a consecutive series of 1,000
cases. J Urol 2001; 166:1729.
21.
Andriole, GL, Smith, DS, Rao, G, et al. Early complications of
contemporary anatomical radical retropubic prostatectomy. J Urol 1994;
152:1858.
22.
Nuttall, GA, Cragun, MD, Hill, DL, et al. Radical retropubic prostatectomy
and blood transfusion. Mayo Clin Proc 2002; 77:1301.
23.
Lerner, SE, Blute, ML, Lieber, MM, Zincke, H. Morbidity of contemporary
radical retropubic prostatectomy for localized prostate cancer. Oncology
(Huntingt) 1995; 9:379.
24.
Dash, A, Dunn, RL, Resh, J, et al. Patient, surgeon, and treatment
characteristics associated with homologous blood transfusion requirement during
radical retropubic prostatectomy: multivariate nomogram to assist patient
counseling. Urology 2004; 64:117.
25.
Costello, AJ, Brooks, M, Cole, OJ. Anatomical studies of the
neurovascular bundle and cavernosal nerves. BJU Int 2004; 94:1071.
26.
Sofer, M, Hamilton-Nelson, KL, Schlesselman, JJ, Soloway, MS. Risk of
positive margins and biochemical recurrence in relation to nerve-sparing radical
prostatectomy. J Clin Oncol 2002; 20:1853.
27.
Ward, JF, Zincke, H, Bergstralh, EJ, et al. The impact of surgical approach
(nerve bundle preservation versus wide local excision) on surgical margins and
biochemical recurrence following radical prostatectomy. J Urol 2004; 172:1328.
28.
Kamat, AM, Jacobsohn, KM, Troncoso, P, et al. Validation of criteria used
to predict extraprostatic cancer extension: a tool for use in selecting patients for
nerve sparing radical prostatectomy. J Urol 2005; 174:1262.
29.
Ogura, K, Maekawa, S, Okubo, K, et al. Dynamic endorectal magnetic
resonance imaging for local staging and detection of neurovascular bundle
involvement of prostate cancer: correlation with histopathologic results. Urology
2001; 57:721.
30.
Hricak, H, Wang, L, Wei, DC, et al. The role of preoperative endorectal
magnetic resonance imaging in the decision regarding whether to preserve or
resect neurovascular bundles during radical retropubic prostatectomy. Cancer
2004; 100:2655.
31.
Epstein, JI, Pizov, G, Walsh, PC. Correlation of pathologic findings with
progression after radical retropubic prostatectomy. Cancer 1993; 71:3582.
32.
Rosen, MA, Goldstone, L, Lapin, S, et al. Frequency and location of
extracapsular extension and positive surgical margins in radical prostatectomy
specimens. J Urol 1992; 148:331.
33.
Epstein, JI. Evaluation of radical prostatectomy capsular margins of
resection. The significance of margins designated as negative, closely
approaching, and positive. Am J Surg Pathol 1990; 14:626.
34.
Cangiano, TG, Litwin, MS, Naitoh, J, et al. Intraoperative frozen section
monitoring of nerve sparing radical retropubic prostatectomy. J Urol 1999;
162:655.
35.
Kim, ED, Scardino, PT, Hampel, O, et al. Interposition of sural nerve
restores function of cavernous nerves resected during radical prostatectomy. J
Urol 1999; 161:188.
36.
Secin, FP, Koppie, TM, Scardino, PT, et al. Bilateral cavernous nerve
interposition grafting during radical retropubic prostatectomy: Memorial SloanKettering Cancer Center experience. J Urol 2007; 177:664.
37.
Nelson, BA, Chang, SS, Cookson, MS, Smith, JA Jr. Morbidity and
efficacy of genitofemoral nerve grafts with radical retropubic prostatectomy.
Urology 2006; 67:789.
38.
Klein, EA, Grass, JA, Calabrese, DA, et al. Maintaining quality of care and
patient satisfaction with radical prostatectomy in the era of cost containment.
Urology 1996; 48:269.
39.
Walsh, PC, Partin, AW, Epstein, JI. Cancer control and quality of life
following anatomical radical retropubic prostatectomy: results at 10 years. J Urol
1994; 152:1831.
40.
Zincke, H, Bergstralh, EJ, Blute, ML, et al. Radical prostatectomy for
clinically localized prostate cancer: long-term results of 1,143 patients from a
single institution. J Clin Oncol 1994; 12:2254.
41.
Gerber, GS, Thisted, RA, Scardino, PT, et al. Results of radical
prostatectomy in men with clinically localized prostate cancer. JAMA 1996;
276:615.
42.
Catalona, WJ, Smith, DS. Cancer recurrence and survival rates after
anatomic radical retropubic prostatectomy for prostate cancer: intermediate-term
results. J Urol 1998; 160:2428.
43.
Kupelian, PA, Katcher, J, Levin, HS, Klein, EA. Stage T1-2 prostate
cancer: a multivariate analysis of factors affecting biochemical and clinical
failures after radical prostatectomy. Int J Radiat Oncol Biol Phys 1997; 37:1043.
44.
Clark, PE, Levin, HS, Kupelian, PA, et al. Intermediate-term outcome with
radical prostatectomy for localized prostate cancer. Prostate J 2001; 3:118.
45.
Stein, A, deKernion, JB, Smith, RB, et al. Prostate specific antigen levels
after radical prostatectomy in patients with organ confined and locally extensive
prostate cancer. J Urol 1992; 147:942.
46.
Cookson, MS, Aus, G, Burnett, AL, et al. Variation in the definition of
biochemical recurrence in patients treated for localized prostate cancer: the
American Urological Association Prostate Guidelines for Localized Prostate
Cancer Update Panel report and recommendations for a standard in the
reporting of surgical outcomes. J Urol 2007; 177:540.
47.
Jhaveri, FM, Zippe, CD, Klein, EA, Kupelian, PA. Biochemical failure does
not predict overall survival after radical prostatectomy for localized prostate
cancer: 10-year results. Urology 1999; 54:884.
48.
Pound, CR, Partin, AW, Eisenberger, MA, et al. Natural history of
progression after PSA elevation following radical prostatectomy. JAMA 1999;
281:1591.
49.
Roberts, SG, Blute, ML, Bergstralh, EJ, et al. PSA doubling time as a
predictor of clinical progression after biochemical failure following radical
prostatectomy for prostate cancer. Mayo Clin Proc 2001; 76:576.
50.
Mann, MJ, DeCastro, GJ, Desai, M, et al. Predictive significance of
surgical margin status after prostatectomy for prostate cancer during PSA era.
Urology 2008; 72:1203.
51.
Stephenson, AJ, Kattan, MW, Eastham, JA, et al. Prostate cancer-specific
mortality after radical prostatectomy for patients treated in the prostate-specific
antigen era. J Clin Oncol 2009; 27:4300.
52.
D'Amico, AV, Chen, MH, Roehl, KA, Catalona, WJ. Preoperative PSA
velocity and the risk of death from prostate cancer after radical prostatectomy. N
Engl J Med 2004; 351:125.
53.
Vickers, AJ, Bianco, FJ, Serio, AM, et al. The surgical learning curve for
prostate cancer control after radical prostatectomy. J Natl Cancer Inst 2007;
99:1171.
54.
Klein, EA, Bianco, FJ, Serio, AM, et al. Surgeon experience is strongly
associated with biochemical recurrence after radical prostatectomy for all
preoperative risk categories. J Urol 2008; 179:2212.
55.
Shariat, SF, Karakiewicz, PI, Roehrborn, CG, Kattan, MW. An updated
catalog of prostate cancer predictive tools. Cancer 2008; 113:3075.
56.
Dillioglugil, O, Leibman, BD, Leibman, NS, et al. Risk factors for
complications and morbidity after radical retropubic prostatectomy. J Urol 1997;
157:1760.
57.
Alibhai, SM, Leach, M, Tomlinson, G, et al. 30-day mortality and major
complications after radical prostatectomy: influence of age and comorbidity. J
Natl Cancer Inst 2005; 97:1525.
58.
Begg, CB, Riedel, ER, Bach, PB, et al. Variations in morbidity after radical
prostatectomy. N Engl J Med 2002; 346:1138.
59.
Wilt, TJ, Shamliyan, TA, Taylor, BC, et al. Association between hospital
and surgeon radical prostatectomy volume and patient outcomes: a systematic
review. J Urol 2008; 180:820.
60.
Shelfo, SW, Obek, C, Soloway, MS. Update on bladder neck preservation
during radical retropubic prostatectomy: impact on pathologic outcome,
anastomotic strictures, and continence. Urology 1998; 51:73.
61.
Licht, MR, Klein, EA, Tuason, L, Levin, H. Impact of bladder neck
preservation during radical prostatectomy on continence and cancer control.
Urology 1994; 44:883.
62.
Sanda, MG, Dunn, RL, Michalski, J, et al. Quality of life and satisfaction
with outcome among prostate-cancer survivors. N Engl J Med 2008; 358:1250.
63.
Kundu, SD, Roehl, KA, Eggener, SE, et al. Potency, continence and
complications in 3,477 consecutive radical retropubic prostatectomies. J Urol
2004; 172:2227.
64.
Goluboff, ET, Saidi, JA, Mazer, S. Urinary continence after radical
prostatectomy: the Columbia experience. J Urol 1998; 159:1276.
65.
Wei, JT, Dunn, RL, Marcovich, R, et al. Prospective assessment of patient
reported urinary continence after radical prostatectomy. J Urol 2000; 164:744.
66.
Stanford, JL, Feng, Z, Hamilton, AS, et al. Urinary and sexual function
after radical prostatectomy for clinically localized prostate cancer: the Prostate
Cancer Outcomes Study. JAMA 2000; 283:354.
67.
Benoit, RM, Naslund, MJ, Cohen, JK. Complications after radical
retropubic prostatectomy in the medicare population. Urology 2000; 56:116.
68.
Litwin, MS, Pasta, DJ, Yu, J, et al. Urinary function and bother after radical
prostatectomy or radiation for prostate cancer: a longitudinal, multivariate quality
of life analysis from the Cancer of the Prostate Strategic Urologic Research
Endeavor. J Urol 2000; 164:1973.
69.
Potosky, AL, Legler, J, Albertsen, PC, et al. Health outcomes after
prostatectomy or radiotherapy for prostate cancer: results from the prostate
cancer outcomes study. J Natl Cancer Inst 2000; 92:1582.
70.
Moore, KN, Cody, DJ, Glazener, CM. Conservative management of post
prostatectomy incontinence. Cochrane Database Syst Rev 2000; :CD001843.
71.
Hunter, KF, Glazener, CM, Moore, KN. Conservative management for
postprostatectomy urinary incontinence. Cochrane Database Syst Rev 2007;
:CD001843.
72.
Van Kampen, M, De Weerdt, W, Van Poppel, H, et al. Effect of pelvic-floor
re-education on duration and degree of incontinence after radical prostatectomy:
a randomised controlled trial. Lancet 2000; 355:98.
73.
Wille, S, Sobottka, A, Heidenreich, A, Hofmann, R. Pelvic floor exercises,
electrical stimulation and biofeedback after radical prostatectomy: results of a
prospective randomized trial. J Urol 2003; 170:490.
74.
Comiter, CV. Surgery insight: surgical management of prostatectomy
incontinence - the artificial urinary sphincter and male sling. Nat Clin Pract Urol
2007; 4:615.
75.
Kuznetsov, DD, Kim, HL, Patel, RV, et al. Comparison of artificial urinary
sphincter and collagen for the treatment of postprostatectomy incontinence.
Urology 2000; 56:600.
76.
Smith, DN, Appell, RA, Rackley, RR, Winters, JC. Collagen injection
therapy for post-prostatectomy incontinence. J Urol 1998; 160:364.
77.
Sanchez-Ortiz, RF, Broderick, GA, Chaikin, DC, et al. Collagen injection
therapy for post-radical retropubic prostatectomy incontinence: role of Valsalva
leak point pressure. J Urol 1997; 158:2132.
78.
Elliott, DS, Barrett, DM. Mayo Clinic long-term analysis of the functional
durability of the AMS 800 artificial urinary sphincter: a review of 323 cases. J Urol
1998; 159:1206.
79.
Comiter, CV. The male perineal sling: intermediate-term results. Neurourol
Urodyn 2005; 24:648.
80.
Rajpurkar, AD, Onur, R, Singla, A. Patient satisfaction and clinical efficacy
of the new perineal bone-anchored male sling. Eur Urol 2005; 47:237.
81.
Fischer, MC, Huckabay, C, Nitti, VW. The male perineal sling: assessment
and prediction of outcome. J Urol 2007; 177:1414.
82.
Elliott, SP, Meng, MV, Elkin, EP, et al. Incidence of urethral stricture after
primary treatment for prostate cancer: data From CaPSURE. J Urol 2007;
178:529.
83.
Montgomery, JS, Gayed, BA, Daignault, S, et al. Early urinary retention
after catheter removal following radical prostatectomy predicts for future
symptomatic urethral stricture formation. Urology 2007; 70:324.
84.
Yurkanin, JP, Dalkin, BL, Cui, H. Evaluation of cold knife urethrotomy for
the treatment of anastomotic stricture after radical retropubic prostatectomy. J
Urol 2001; 165:1545.
85.
Walsh, PC, Marschke, P, Ricker, D, Burnett, AL. Patient-reported urinary
continence and sexual function after anatomic radical prostatectomy. Urology
2000; 55:58.
86.
Mettlin, CJ, Murphy, GP, Sylvester, J, et al. Results of hospital cancer
registry surveys by the American College of Surgeons: outcomes of prostate
cancer treatment by radical prostatectomy. Cancer 1997; 80:1875.
87.
Saranchuk, JW, Kattan, MW, Elkin, E, et al. Achieving optimal outcomes
after radical prostatectomy. J Clin Oncol 2005; 23:4146.
88.
Raina, R, Agarwal, A, Zippe, CD. Management of erectile dysfunction after
radical prostatectomy. Urology 2005; 66:923.
89.
Schover, LR, Fouladi, RT, Warneke, CL, et al. The use of treatments for
erectile dysfunction among survivors of prostate carcinoma. Cancer 2002;
95:2397.
90.
Zippe, CD, Jhaveri, FM, Klein, EA, et al. Role of viagra after radical
prostatectomy. Urology 2000; 55:241.
91.
Feng, MI, Huang, S, Kaptein, J, et al. Effect of sildenafil citrate on postradical prostatectomy erectile dysfunction. J Urol 2000; 164:1935.
92.
Lowentritt, BH, Scardino, PT, Miles, BJ, et al. Sildenafil citrate after radical
retropubic prostatectomy. J Urol 1999; 162:1614.
93.
Hong, EK, Lepor, H, McCullough, AR. Time dependent patient satisfaction
with sildenafil for erectile dysfunction (ED) after nerve-sparing radical retropubic
prostatectomy (RRP). Int J Impot Res 1999; 11 Suppl 1:S15.
94.
Padma-Nathan, H, McCullough, AR, Levine, LA, et al. Randomized,
double-blind, placebo-controlled study of postoperative nightly sildenafil citrate
for the prevention of erectile dysfunction after bilateral nerve-sparing radical
prostatectomy. Int J Impot Res 2008; 20:479.
95.
Montorsi, F, Brock, G, Lee, J, et al. Effect of nightly versus on-demand
vardenafil on recovery of erectile function in men following bilateral nerve-sparing
radical prostatectomy. Eur Urol 2008; 54:924.
96.
Lepor, H, Robbins, D. Inguinal hernias in men undergoing open radical
retropubic prostatectomy. Urology 2007; 70:961.
97.
Sun, M, Lughezzani, G, Alasker, A, et al. Comparative Study of Inguinal
Hernia Repair After Radical Prostatectomy, Prostate Biopsy, Transurethral
Resection of the Prostate or Pelvic Lymph Node Dissection. J Urol 2010;
183:970.
98.
Matsubara, A, Yoneda, T, Nakamoto, T, et al. Inguinal hernia after radical
perineal prostatectomy: comparison with the retropubic approach. Urology 2007;
70:1152.
99.
Slova, D, Lepor, H. The short-term and long-term effects of radical
prostatectomy on lower urinary tract symptoms. J Urol 2007; 178:2397.
100.
Hu, JC, Gu, X, Lipsitz, SR, et al. Comparative effectiveness of minimally
invasive vs open radical prostatectomy. JAMA 2009; 302:1557.
101.
Smith, JA Jr, Chan, RC, Chang, SS, et al. A comparison of the incidence
and location of positive surgical margins in robotic assisted laparoscopic radical
prostatectomy and open retropubic radical prostatectomy. J Urol 2007; 178:2385.
102.
Blute, ML. Radical prostatectomy by open or laparoscopic/robotic
techniques: an issue of surgical device or surgical expertise?. J Clin Oncol 2008;
26:2248.
103.
Guillonneau, B, el-Fettouh, H, Baumert, H, et al. Laparoscopic radical
prostatectomy: oncological evaluation after 1,000 cases a Montsouris Institute. J
Urol 2003; 169:1261.
104.
Badani, KK, Kaul, S, Menon, M. Evolution of robotic radical prostatectomy:
assessment after 2766 procedures. Cancer 2007; 110:1951.
105.
Hu, JC, Wang, Q, Pashos, CL, et al. Utilization and outcomes of minimally
invasive radical prostatectomy. J Clin Oncol 2008; 26:2278.
106.
Hu, JC, Hevelone, ND, Ferreira, MD, et al. Patterns of care for radical
prostatectomy in the United States from 2003 to 2005. J Urol 2008; 180:1969.
107.
Wood, DP, Schulte, R, Dunn, RL, et al. Short-term health outcome
differences between robotic and conventional radical prostatectomy. Urology
2007; 70:945.
108.
Hull, GW, Rabbani, F, Abbas, F, et al. Cancer control with radical
prostatectomy alone in 1,000 consecutive patients. J Urol 2002; 167:528.
109.
Weizer, AZ, Ye, Z, Hollingsworth, JM, et al. Adoption of new technology
and healthcare quality: surgical margins after robotic prostatectomy. Urology
2007; 70:96.
110.
Vickers, AJ, Savage, CJ, Hruza, M, et al. The surgical learning curve for
laparoscopic radical prostatectomy: a retrospective cohort study. Lancet Oncol
2009; 10:475.
111.
Martis, G, Diana, M, Ombres, M, et al. Retropubic versus perineal radical
prostatectomy in early prostate cancer: eight-year experience. J Surg Oncol
2007; 95:513.
112.
Iselin, CE, Robertson, JE, Paulson, DF. Radical perineal prostatectomy:
oncological outcome during a 20-year period. J Urol 1999; 161:163.
113.
Lance, RS, Freidrichs, PA, Kane, C, et al. A comparison of radical
retropubic with perineal prostatectomy for localized prostate cancer within the
Uniformed Services Urology Research Group. BJU Int 2001; 87:61.
114.
Partin, AW, Yoo, J, Carter, HB, et al. The use of prostate specific antigen:
Clinical stage and Gleason score to predict pathological stage in men with
localized prostate cancer. J Urol 1993; 150:110.
115.
Partin, AW, Kattan, MW. Combination of prostate-specific antigen, clinical
stage, and Gleason score to predict pathological stage of localized prostate
cancer. A multi-institutional update. JAMA 1997; 277:1445.
116.
Gleave, ME, Goldenberg, SL, Jones, EC, et al. Biochemical and
pathological effects of 8 months of neoadjuvant androgen withdrawal therapy
before radical prostatectomy in patients with clinically confined prostate cancer
[see comments]. J Urol 1996; 155:213.
117.
Gleave, ME, La Bianca, SE, Goldenberg, SL, et al. Long-term neoadjuvant
hormone therapy prior to radical prostatectomy: evaluation of risk for biochemical
recurrence at 5-year follow-up. Urology 2000; 56:289.
118.
Soloway, MS, Pareek, K, Sharifi, R, Wajsman, Z. Neoadjuvant androgen
ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5-year
results. J Urol 2002; 167:112.
119.
Schulman, CC, Debruyne, FM, Forster, G, et al. 4-year follow-up results of
a European prospective randomized study on neoadjuvant hormonal therapy
prior to radical prostatectomy in T2-3N0M0 prostate cancer. Eur Urol 2000;
38:706.
120.
Klotz, LH, Goldenberg, SL, Jewett, MA, et al. Long-term followup of a
randomized trial of 0 versus 3 months of neoadjuvant androgen ablation before
radical prostatectomy. J Urol 2003; 170:791.
121.
Clark, PE, Peereboom, DM, Dreicer, R, et al. Phase II trial of neoadjuvant
estramustine and etoposide plus radical prostatectomy for locally advanced
prostate cancer. Urology 2001; 57:281.
122.
Pettaway, CA, Pisters, LL, Troncoso, P, et al. Neoadjuvant chemotherapy
and hormonal therapy followed by radical prostatectomy: feasibility and
preliminary results. J Clin Oncol 2000; 18:1050.
123.
Febbo, PG, Richie, JP, George, DJ, et al. Neoadjuvant docetaxel before
radical prostatectomy in patients with high-risk localized prostate cancer. Clin
Cancer Res 2005; 11:5233.
124.
Garzotto, M, Myrthue, A, Higano, CS, Beer, TM. Neoadjuvant
mitoxantrone and docetaxel for high-risk localized prostate cancer. Urol Oncol
2006; 24:254.
125.
Beer, TM, Garzotto, M, Lowe, BA, et al. Phase I study of weekly
mitoxantrone and docetaxel before prostatectomy in patients with high-risk
localized prostate cancer. Clin Cancer Res 2004; 10:1306.
126.
Magi-Galluzzi, C, Zhou, M, Reuther, AM, et al. Neoadjuvant docetaxel
treatment for locally advanced prostate cancer: a clinicopathologic study. Cancer
2007; 110:1248.
127.
Winquist, E, et al. Multicenter phase II study. of combined neoadjuvant
docetaxel and androgen ablation (ADT) prior to radical prostatectomy for patients
with high risk localized prostate cancer: pathologic outcomes and 3-yeare followup analysis (abstract). J Clin Oncol 2007; 25:235s. (Abstract available online at
www.asco.org/portal/site/ASCO/menuitem.34d60f5624ba07fd506fe310ee37a01d
/?vgnextoid=76f8201eb61a7010VgnVCM100000ed730ad1RCRD&vmview=abst
_detail_view&confID=47&abstractID=30613, accessed June 18, 2007).
128.
Iversen, P, McLeod, DG, See, WA, et al. Antiandrogen monotherapy in
patients with localized or locally advanced prostate cancer: final results from the
bicalutamide Early Prostate Cancer programmer at a median follow-up of 9.7
years. BJUI 2010; 105:1074.
© 2010 UpToDate, Inc. All rights reserved.