ABn molecules
advertisement
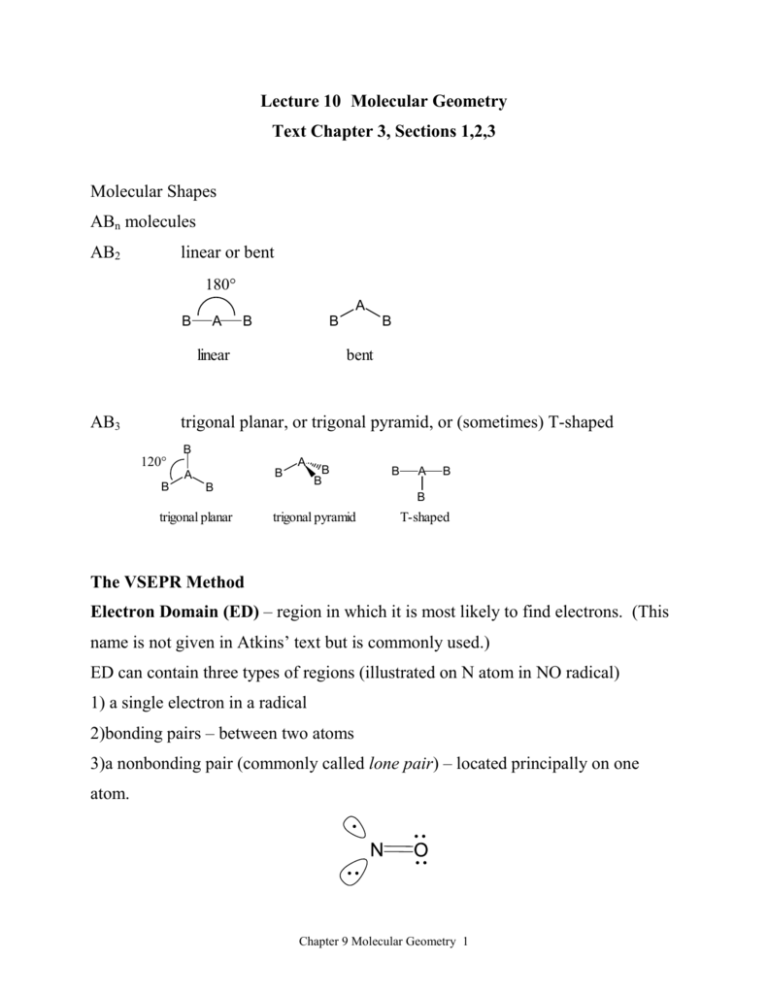
Lecture 10 Molecular Geometry Text Chapter 3, Sections 1,2,3 Molecular Shapes ABn molecules AB2 linear or bent 180° A B A B B linear AB3 B bent trigonal planar, or trigonal pyramid, or (sometimes) T-shaped 120° B A B A B B trigonal planar B B B A B B trigonal pyramid T-shaped The VSEPR Method Electron Domain (ED) – region in which it is most likely to find electrons. (This name is not given in Atkins’ text but is commonly used.) ED can contain three types of regions (illustrated on N atom in NO radical) 1) a single electron in a radical 2)bonding pairs – between two atoms 3)a nonbonding pair (commonly called lone pair) – located principally on one atom. Chapter 9 Molecular Geometry 1 1)The best arrangement of a given number of electron domains is the one that minimizes the repulsions among them. 2)”Bond Regions” contain single, double or triple bonds 3a) The arrangement of electron domains about the central atom of an ABn molecule is called its electron-domain geometry (called in textbook, “regions of high electron concentration”. Number of Arrangement of Electron Predicted electron domain bond geometry angles linear 180° trigonal 120° electron domains domains 2 B A B 3 B 4 A planar B B 109.5° B A B B tetrahedral 109.5° B Chapter 9 Molecular Geometry 2 5 6 B 120° B B A B 90° trigonal 120° B bipyramid 90° B B B A B B B octahedral 90° 180° 3b) The arrangement of the atoms (nuclei) of a molecule in space is the “molecular geometry” (sometimes called “molecular shape”). Steps to predict molecular geometries 1. Sketch the Lewis structure 2. Count the total number of electron domains around the central atom, and arrange them in a way to minimize repulsions. 3. Describe the molecular geometry in terms of the angular arrangement of the bonded atoms. Chapter 9 Molecular Geometry 3 4. A double or triple bond is counted as one electron domain when predicting geometry. (# of electron domains) = (# of atoms bonded to central atom) + (# of nonbonding pairs on the central atom) The effect of nonbonding electrons and multiple bonds on bond angles. Electron domains for nonbonding electron pairs exert greater repulsive forces on adjacent electron domains and thus tend to compress the bond angles. H C H H H 109.5° H H N O H 107° H H 104.5° Electron domains for multiple bonds exert a greater repulsive force on adjacent electron domains than do single bonds. Chapter 9 Molecular Geometry 4 124.3° Cl C 111.4° O Cl 124.3° “Normal” angle = 120o Molecules with expanded valence shells. Atoms from the third period and beyond can have more than four pairs of electrons around them. Five electron domains give rise to trigonal bipyramidal electron domain geometries. Trigonal bipyramidal structures have three electron domains in the equatorial position and two in the axial positions. Axials have three 90° interactions while equatorial positions have only two. Put the (l.p.) bulkier domains in the equatorial positions first. Chapter 9 Molecular Geometry 5 Six electron domains give rise to octahedral geometries. All electron domains are at 90° to four other electron domains. Coordination Electronic Number Nonbonding Molecular Geometry Geometry Pairs 2 linear 0 linear 3 trigonal planar 0 trigonal planar 1 bent 0 tetrahedral 1 trigonal pyramid 2 bent 0 trigonal bipyramid 1 see-saw 2 T-shaped 3 linear 0 octahedral 1 square pyramid 2 square planar 4 5 6 tetrahedral trigonal bipyramid octahedral Chapter 9 Molecular Geometry 6 trigonal linear trigonal bipyramid tetrahedral octahedral F O C N O O O O Xe H H F F F B F F N H H F Cl H F F H Si H H F F H F S F Xe F F F Cl F Cl Cl P Cl F Br F F F Cl F F F S F F F Chapter 9 Molecular Geometry 7 Dr. Burke's Quick Method (must first be familiar with valency of C,N,O atoms) Number of El. Domains on atom = Group No. + No. “pure” single bonds – No. triple bonds – charge 2 Number of LP on atom = No. ED – No. bond regions Examples: – 1) EDs on Sb atom in SbF4 = 5 + 4 - 0 - (-1) =5 2 Trigonal bipyramid electronic geometry. 5 EDs - 4 bond regions = one lone pair. F F Sb F F F 3 x 90° Sb F F F 2 x 90 + 2 x 120 Molecular geometry: see-saw (NOT trigonal pyramid shown on left) 2) EDs on S atom in SO2 = (Remember that the valency on O atoms with only one partner is 2. So double bond or bond in resonance.) LP = ED – Bond Regions = 3 – 2 = 1 Chapter 9 Molecular Geometry 8 3) EDs on S atom in SO32- = LP = ED – Bond Regions = 4 – 3 = 1 4) EDs on S atom in SO42- = LP = ED – Bond Regions = 4 – 4 = 0 5)`EDs on S atom in HSO4- = LP = ED – Bond Regions = 4 – 4 = 0 Chapter 9 Molecular Geometry 9 Molecules with more than one central atom. O H H C C H O H Polarity in Polyatomic Molecules Chapter 9 Molecular Geometry 10 O O H C O N Cl H Cl H H B Cl Cl Cl Cl Cl H H C Cl H H Dipole Moment = vector sum of bond moments Chapter 9 Molecular Geometry 11 C H Cl