Biological Molecules
advertisement

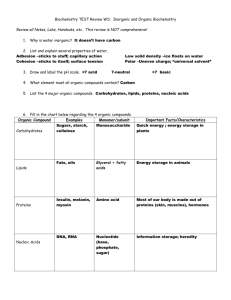
Lecture 6 Biological Molecules Basic chemistry Group of molecules especially important Organic compounds: carbon compounds Some books refer to organic compounds as being formed by living things: too simplistic Biologically Important Molecules Some molecules essential for life W ater Carbohydrates Lipids Proteins Nucleic Acids W ater W ater important: Source of H+ and OH Solvent—important elements are carried by water through the biotic elements Polar–attracted to other water molecules High specific heat—can absorb large amount of heat without changing state Insulator W ater Evaporative processes; increase in molecular motion, molecules escape, surface cools Sphere of hydration: formation of useful ions Heat distribution on a global scale: evaporating 1 g water absorbs 580 cal Carbon Compounds Carbon: forms 4 covalent bonds Orientation of atoms along C backbone 3 dimensional, not flat Organic Compounds Electron transfer Functional group transfer Bond rearrangement Condensation: 2 molecules combine with covalent bonds Cleavage: molecule splits into 2 smaller, hydrolysis (water splits, H, OH) Organic Compounds Carbon backbone Single unit: monomer Many units: polymer Many molecules are polymers Organic Compounds Functional groups: groups of atoms attached to C backbone Solubility Reactivity Binding sites Carbohydrates Most abundant organic Simple or complex Simple energy source for cells Stored energy Cell wall components W ater soluble C, H, O: 1:2:1 ratio Carbohydrates Building block: simple sugar—saccharide Monosaccharide: 1 sugar unit Glucose: primary energy source Short chains: few repeating units—disachharide, sucrose Oligosaccharide: with protein, found on cell membranes Carbohydrates Complex carbohydrates: many unnits covalently bonded Starch Cellulose Glycogen Chitin Lipids C, H, O: not 1:2:1 ratio Fats Many uses, include high density energy source (9 cal/g vs 4 cal/g) Common lipids Glycerides Steroids Phospholipids Lipids Glyceride: glycerol (head) and fatty acids (tails) Saturated: no double bonds Unsaturated: double bonds Polyunsaturated: many double bonds Lipids Steroids 4 carbon rings Many different side chains Hormones Cell components, especially cell membrane Proteins C, H, O , N, S Building blocks: amino acids 3+: polypeptide Amino group and acid groups 20 different amino acids, great variety of proteins Proteins Primary structure: aa sequence Secondary structure: H bonds form spirals or pleats Tertiary Structure: folding of chains (proteins) Tertiary structure: 2 or more chains joined (subunits) Proteins Krogh, p. 48, Table 3.2 Lists: T ypes—enzymes, hormones, etc Roles—biochemical catalysts, messengers Examples—am ylase (lab), insulin Proteins Functional groups essential Shape is essential Alter shape, alter function Heat pH Nucleotides and Nucleic Acids C, H, O , N, P Chemical energy carriers Storage and transfer of genetic information and protein formation Nucleotide Phosphate group Sugar group Nitrogenous base DNA is like a cookbook Nucleic Acids Each recipe (“gene”) calls for the production of a particular protein RNA is involved in bringing DNA’s instructions to the site of protein synthesis