Salt, Sand, Iron Mixture: Percent Composition Lab
advertisement

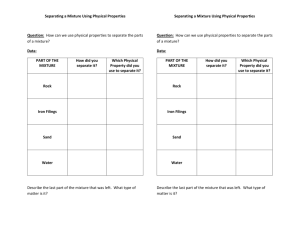
Lab 2 Name_____________________________ How Can the Percent Composition of a Salt, Sand and Iron Mixture be Determined? Pre-Lab Assignment This written pre-lab is worth 15% (3 points) of your lab report grade and must be turned in to your lab instructor before lab begins. 1. 2. 3. 4. Read the entire lab handout. Make a list of all chemicals used in this lab (may be names or chemical formulas). List the suspected safety hazards in today’s lab AND the safety precautions that should be taken to protect yourself from these hazards. Put this list in a table: one column for safety hazards, one for related safety precautions. Write a brief summary (2-3 sentences each) of each experiment that will be performed today (there are two – one for each experimental question). Hints: Do not just list each step of the procedure, but practice your summarizing skills. For example, the Background section states, “To get the numerical percent of salt in the mixture, you need to determine the mass of salt and the mass of the mixture you obtained that salt from.” Explain in one or two sentences how you will get that data. Experimental Question How can the percent composition of a salt, sand and iron mixture be determined? Learning goals Make detailed qualitative observations in your data record. Make metric measurements with proper units. Use different types of glassware and understand their functions. Use a Bunsen burner properly. Investigate the principle behind filtration. Use different methods for separating different kinds of mixtures. Background: Scientific Method Scientific inquiry (also called the scientific method) is a process that scientists use to explore the natural world and propose explanations based on the evidence they find. The main purpose of the CH 104 lab is to give you experience doing scientific inquiry on concepts covered in the CH 104 course. You will be practicing the scientific method through a series of steps each week. Your Mastering Chemistry tutorial on the scientific method has a good description of the scientific method: The scientific method is a procedure used to search for explanations of nature. The scientific method consists of making observations, formulating hypotheses, designing and carrying out experiments, and repeating this cycle. Observations can be either quantitative or qualitative. Quantitative observations are measurements consisting of both numbers and units, such as the observation that ice melts at 0ºC. In contrast, qualitative observations are observations that do not rely on numbers or units, such as the observation that water is clear. A hypothesis is a tentative explanation of the observations. The hypothesis is not necessarily correct, but it puts the scientist's understanding of the observations into a form that can be tested through experimentation. Experiments are then performed to test the validity of the hypothesis. Experiments are observations preferably made under conditions in which the variable of interest is clearly distinguishable from any others. If the experiment shows that the hypothesis is incorrect, the hypothesis can be modified, and further experiments can be carried out to test the modified hypothesis. This cycle is repeated, continually refining the hypothesis. If a large set of observations follow a reproducible pattern, this pattern can be summarized in a law—a verbal or mathematical generalization of a phenomenon. For example, over the years people observed that every morning the sun rises in the east, and every night the sun sets in the west. These observations can be described in a law stating, "The sun always rises in the east and sets in the west." After a great deal of refinement, a hypothesis can lead to a theory. A theory is an explanation of why something happens. For example, Newton's theory of gravitation explains why objects tend to fall toward the Earth (as well as explaining the interactions between the Earth and the other planets, etc). However, theories can still be further refined or even replaced. Einstein's theory of general relativity was able to better explain certain astronomical observations related to gravity, and therefore it replaced Newton's theory of gravitation (although Newton's theory still holds true under most everyday Lab 2: How Can the Percent Composition of a Salt, Sand and Iron Mixture be Determined 2 conditions). Similarly, the geocentric theory (that the Earth is the center of the universe) was replaced by the heliocentric theory (that the Earth revolves around the sun) based on further observations and testing of predictions. Note that a scientific theory is not the same as the popular definition of a theory—namely, a "guess" or "speculation." Instead, a theory is an explanation that can hold up against repeated experimentation. It may not be perfect, but it is the best explanation possible based on available evidence. The Experiments portion of the scientific method given above can be broken down even further: Experimental question: should be answered by the experiments. Procedural plan: takes into account many factors such as changing only one variable at a time while controlling all other variables, performing multiple trials, considering limitations of time and materials, running a control, etc. Data collection: includes using appropriate glassware and instruments to make accurate quantitative measurements with correct significant figures, making qualitative observations, and organizing your data into a data table. All data collected in the lab must be recorded directly onto your paper at the time of the observation. There will be no measurements on scratch paper or re-copying direct data later. Don’t worry – you’ll have the chance to organize your data into a more readable format in the data tables after data collection has been completed. Data Analysis: may include mathematical calculations, graphing, or otherwise manipulating your raw data (measurements) to answer the experimental question. Also includes examination of the level of experimental uncertainty. You may often find your qualitative observations important in drawing conclusions. Conclusion: final result of the data analysis which answers the experimental question. Hypothesis may be confirmed or denied. Background: Qualitative and Quantitative Observations Some experiments provide quantitative data and some provide qualitative data. Quantitative methods are those which focus on numbers and provide information which is easy to analyze statistically and fairly reliably. The measurements you make in lab and record on your data sheet are quantitative, as are the numerical, calculated results of your experimental data. Most scientific experiments are quantitative in nature, though they also possess a qualitative component. Qualitative methods are descriptive; they are concerned with describing meaning, rather than with drawing statistical conclusions. Most of the observations you write in your lab notebook, such as a substance’s color or odor, are qualitative in nature. As a rule of thumb, remember that qualitative data is typically expressed in words while quantitative data is expressed as numbers. Qualitative: Lead is much denser than aluminum. Quantitative: Lead has a density of 11.3g/cm3 while aluminum’s density is 2.7g/cm3. or, One cubic centimeter of lead weighs 11.3g, while the same volume of aluminum weighs only 2.7g. Procedure Please work with a partner; record his/her full name. My lab partner is: ________________________________________ Write down all qualitative and quantitative observations and measurements directly on this sheet. Note that the equipment needed for each experiment is located either in your lab drawer, on the cart in the center of the lab or in a labeled cabinet or drawer. Critical Thinking Question 1: What measurements are needed to calculate the percent of iron in the mixture? Lab 2: How Can the Percent Composition of a Salt, Sand and Iron Mixture be Determined 3 Obtain and make qualitative observations on the mixture as described below. Shake the bottle containing the mixture of salt, sand and iron to mix it well. Obtain about 10 mL of the mixture in a small beaker. Make observations on the mixture. Samples of the mixture are set up under dissecting microscopes in the lab. Examine the mixture under the microscope and record the appearance of the particles you see. Distinguish between the salt crystals, the grains of sand and the iron filings in drawings and your description Record a hypothesis for this experiment (an answer to the experimental question): Critical Thinking Question 2: Do you expect teams to get the same or different percent compositions of the mixture? Explain briefly. Take your beaker containing the salt, sand & iron mixture along with the small flask to the top loading electronic balance and weigh about 5 grams of the mixture according to the instructions below. Mass measurements using a top-loading balance: When using a balance to make mass measurements, be sure all containers and substances are at room temperature. Place a piece of weighing paper on the balance pan. Close the top of the balance. Wait until the reading holds steady and push the “tare” button. The balance should now read “0.000” or “0.00” grams, depending on the precision of the balance. Hold the mouth of the beaker over the weighing paper, then tap on the side of the beaker so that small amounts of the mixture pour onto the paper. Keep adding the mixture until you have a mass between 4.5 and 5.5 grams. Record the actual mass of your sample in grams, including all digits, even zeros at the end. For example, if the balance shows a readout of 5.200, you need to record “5.200 g” on your paper, NOT “5.2 g”. Carefully pour the sample from the paper into the flask. Dispose of the paper in the trash can. m = _____________ g Separate the iron from the mixture: Spread the mixture into a very thin layer over a full sized piece of paper. Weigh and record the mass of a second piece of weighing paper and set it aside. Wrap a small square of clear plastic over the magnet. Remove the iron powder/filings by passing the magnet closely over the surface of the entire mixture. Repeat several times to make sure you’ve collected all the iron. Holding the magnet over the weighing paper, carefully remove the plastic and allow all the iron to fall onto the paper. Weigh and determine the net mass of the iron powder/filings. m = _____________ g Describe the appearance of the mixture after removing the magnetic iron. Lab 2: How Can the Percent Composition of a Salt, Sand and Iron Mixture be Determined 4 At your bench, use the graduated cylinder to measure 25.0 mL of water as precisely as you can (see instructions below). Note that 25.0 is where the liquid is right on the line - use a dropper for the last few drops. Volume measurements using a graduated cylinder: To read the volume: Place you cylinder on a flat surface. Put your eye level with the meniscus meniscus (meniscus: the curve of the liquid surface).Read the volume at the bottom of the meniscus. Pour the water into the flask containing the mixture. Describe the appearance before, during, and after swirling the mixture continuously for 1 minute: Critical Thinking Question 3: What happened to the salt? Where is the salt now? Filter the mixture as follows: Place the funnel in a ring clamp and set the clamp so that the end of the funnel feeds into the mouth of a small beaker. Fold a piece of filter paper into quarters and place into the funnel, forming a cone. Wet the paper with a squirt from a wash bottle to hold the paper in place. Leaving as much solid on the bottom of the flask as possible, transfer the liquid into the funnel by pouring the liquid down a stirring rod. (see instructor demo) The liquid (called the filtrate) should come through the filter with a clear appearance. If necessary repeat the filtration step with new filter paper. Use the 50 mL graduated cylinder to measure the total volume of the filtrate. Record the volume (to 0.1 mL) V =_____________mL Describe the appearance of the liquid filtrate and the solid remaining on the filter paper. Critical Thinking Question 4: Where is the sand now? Where do you think the salt is now? Evaporate the filtrate as follows: Weigh an evaporating dish and record the mass. m = _____________g Pour the filtrate into the evaporating dish. Adjust the clamp so it rests 3-5 inches above the Bunsen burner. Connect the burner to the gas outlet, and have the instructor inspect your set-up before you light the burner. Instructor initials: ______ Wear safety goggles. Watch your hair and clothing, and have proper footwear. Clear the work area around the burner. Light a match and hold it over the end of the burner. Open the bench valve completely. Adjust the flame color to blue by turning the barrel, and adjust the height of the flame using the knob at the base of the burner so that it just touches the bottom of the gauze on the ring clamp. Allow the liquid to come to a boil in the evaporating dish and keep heating until the water completely evaporates. Be careful of splattering. Lab 2: How Can the Percent Composition of a Salt, Sand and Iron Mixture be Determined 5 Turn off the burner by closing the bench valve. Record descriptions before, during and after heating. Critical Thinking Question 5: Once the water has evaporated, is there anything remaining in the evaporating dish? If so, what could it be? Allow the evaporating dish to cool enough so you cannot feel heat rising from it (usually about ten minutes). Use crucible tongs to grasp the evaporating dish while balancing it on the wire gauze and take it to the weighing room. Do not squeeze the tongs to hard. If your sample spills see instructor. Tare the balance to zero and weigh the dish with the salt. Mass = ______________ g Clean-up Rinse out the evaporating dish in the sink. When the ring clamp and gauze have cooled sufficiently, disassemble and return all equipment to the appropriate areas. Leftover dry mixture may be returned to the community area. Wet sand may be discarded into the trash. Rinse the flask, funnel and other glassware with water before returning them to your drawer. Wipe your bench with a wet paper towel. Return your key to the key box. Congratulations – you have just completed the first part of your scientific inquiry investigating different separation techniques. Data Analysis SHOW ALL WORK. Use proper units and significant figures throughout. 1. Record raw data in Data Table: Transfer all the previous measurements to the Data Table below. Do NOT make direct measurements here! This is the FINAL, NEATLY prepared version of your data. A Data Table is used to organize the observations and measurements made during the lab. Be sure to include units. Mass of mixture Mass of iron filings Volume of Filtrate Mass of empty evaporating dish Mass of evaporating dish + residue Lab 2: How Can the Percent Composition of a Salt, Sand and Iron Mixture be Determined 2. Calculate the mass of the salt residue. 3. Calculate the percent of iron in the original sample. 4. Calculate the percent of salt in the original sample. 5. Calculate the percent of sand in the original sample. 6 part x100% whole Note: when doing any calculations, make units cancel. In this case, each mass must be in the same units (e.g. grams). Percent = Results Table. Used to organize and display the end results of your calculations in one place. Mass (g) Percent of mixture Iron filings Salt Sand Total Get Instructor’s Initials and you can leave the lab. 100% Instructor ______ Questions 1. Give an example of qualitative data from this lab. Give an example of quantitative data. In each cases, be specific, using an example from your collected data. You may quote data from previous pages. Qualitative: Quantitative: 2. How confident are you of your results – the percent composition of each component of the mixture? Explain any sources of experimental uncertainty (reasons your numbers may not be completely accurate). Lab 2: How Can the Percent Composition of a Salt, Sand and Iron Mixture be Determined 3. 7 You started with a mixture of salt, sand and iron. a. Iron was the first component removed from the mixture. What unique physical property of iron allows the separation? b. After filtration and evaporation the mixture is separated into two components. What is the physical property of salt that allows this method to work? c. Is sand a pure substance? Explain using your microscope observations (macroscopic observations in lab). d. Is salt (NaCl) a pure substance? Explain. 4. The salt could be seen in the microscope as distinct from the sand. After dissolving in water, the salt reappears after boiling off the water. On the basis of these observations classify the dissolving of salt as either a physical change or a chemical change and explain your reasons. 5. Filter paper has tiny fibers that criss-cross each other leaving tiny holes between the fibers that liquids can go through called pores. Filter paper can be purchased with different pore sizes depending on what you want to filter. Dry salt cannot pass through the filter paper, yet in the experiment you just performed the salt/water mixture passed through the filter paper. Formulate a hypothesis explaining on a particulate level (on the level of atoms, molecules and ions) why it is possible for the water and the dissolved salt to do so, while the sand stays behind. 6. Use specific examples from this lab to explain how it follows (or does not follow) the steps of the scientific method. Lab 2: How Can the Percent Composition of a Salt, Sand and Iron Mixture be Determined 7. 8 Explain how any one aspect of this lab relates to your daily life. [Hint – look at learning goals, techniques used in lab, chemistry concepts explored. For example, how do you separate mixtures in your daily life? Do you use any of the techniques listed here such as evaporation, filtration, or magnetic properties? Do you use any similar glassware or equipment? If so, for what purpose?] Conclusion A. Answer each Experimental Question using grammatically correct English sentences. How can the percent composition of a salt, sand and iron mixture be determined? B. Using grammatically correct English sentences, describe any points about today’s lab that still need clarification for you. Turn in the Entire Packet at the beginning of the next lab period