exam3a
advertisement
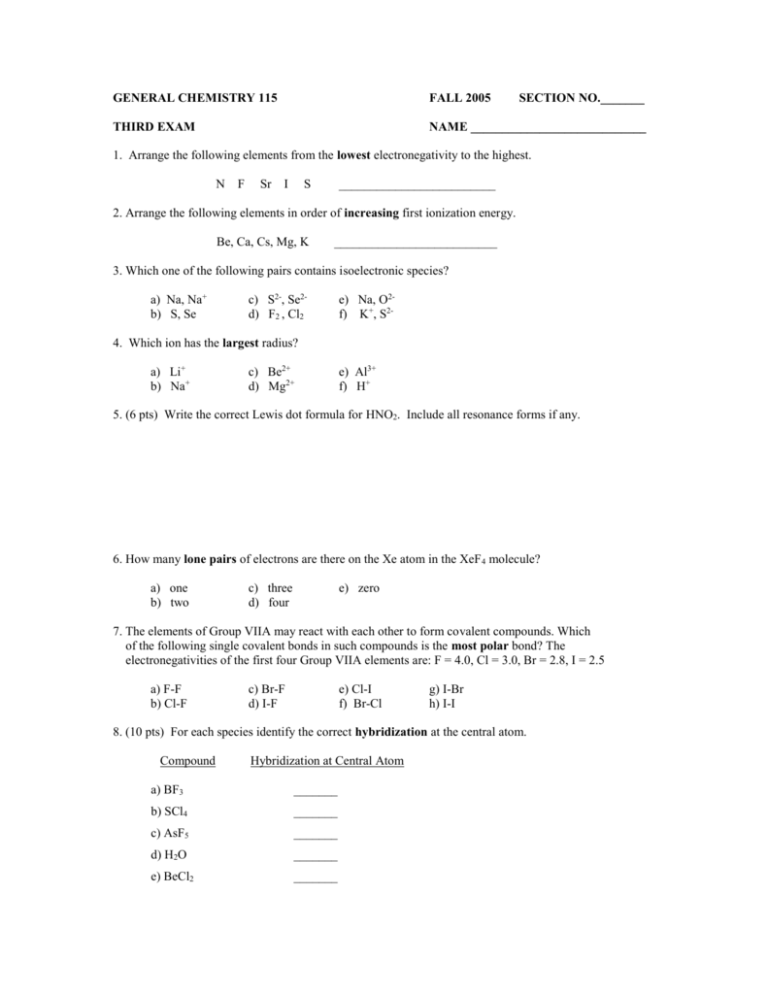
GENERAL CHEMISTRY 115 FALL 2005 SECTION NO._______ THIRD EXAM NAME ____________________________ 1. Arrange the following elements from the lowest electronegativity to the highest. N F Sr I S _________________________ 2. Arrange the following elements in order of increasing first ionization energy. Be, Ca, Cs, Mg, K __________________________ 3. Which one of the following pairs contains isoelectronic species? a) Na, Na+ b) S, Se c) S2-, Se2d) F2 , Cl2 e) Na, O2f) K+, S2- 4. Which ion has the largest radius? a) Li+ b) Na+ c) Be2+ d) Mg2+ e) Al3+ f) H+ 5. (6 pts) Write the correct Lewis dot formula for HNO2. Include all resonance forms if any. 6. How many lone pairs of electrons are there on the Xe atom in the XeF 4 molecule? a) one b) two c) three d) four e) zero 7. The elements of Group VIIA may react with each other to form covalent compounds. Which of the following single covalent bonds in such compounds is the most polar bond? The electronegativities of the first four Group VIIA elements are: F = 4.0, Cl = 3.0, Br = 2.8, I = 2.5 a) F-F b) Cl-F c) Br-F d) I-F e) Cl-I f) Br-Cl g) I-Br h) I-I 8. (10 pts) For each species identify the correct hybridization at the central atom. Compound Hybridization at Central Atom a) BF3 _______ b) SCl4 _______ c) AsF5 _______ d) H2O _______ e) BeCl2 _______ 9. Which, if any, of the compounds listed are not sp3 hybridized at the central atom? I. CF4 II. H2 O III. SiF4 IV. CHCl3 a) III and IV b) I, II, and III V. NH3 c) II, IV, and V d) III and V e) I, III, and V f) all are sp3 hybridized 10. (10 pts) For each of the following pairs of molecules and write the correct molecular geometry. Compound Molecular Geometry a) NF3 ________________ b) H2O ________________ c) BF3 ________________ d) AsF5 ________________ e) SeF6 ________________ 11. Which response contains all the characteristics listed that should apply to phosphorus trichloride, PCl 3, and no other characteristics? I. II. III. IV. V. trigonal planar polar bonds sp3 hybridized at P polar molecule one unshared pair of electrons on P a) I, IV, and V b) II, III, IV and V c) I, II, and IV d) II, IV, and V e) I, II, IV, and V f) none of these 12. Many simple molecules contain lone pairs of electrons (also referred to as unshared pairs) which occupy hybrid orbitals of the central element in a molecule. If an atom of the central element utilizes sp3d hybrid orbitals in a compound, which one of the following types of repulsions would be greatest? a) bonding pair-bonding pair d) lone pair-lone pair b) bonding pair-lone pair e) lone pair-bonding pair c) repulsions between all types of pairs of electrons are the same 13. Arrange the following ionic compounds in order of increasing melting points. NaF , MgCl2 , AlF3 , NaBr , NaI 14. Which of these aqueous solutions would be expected to have the highest boiling point? a) 0.100 m CaCl2 b) 0.200 m NaOH c) 0.050 m K2SO4 d) 0.100 m Al2(SO4)3 e) 0.200 m CH3OH f) 0.300 m CH3COOH 15. A sketch of the phase diagram (not to scale) of water is given below. Which statement is false? a) Line AD is the sublimation curve - solid and vapor are in equilibrium. b) Point A is the triple point - solid, liquid, and vapor are at equilibrium. c) Line AC is the vapor pressure curve - liquid and gas (vapor) are in equilibrium. d) Line AB is the melting curve - solid and liquid are in equilibrium. e) The slope of line AB is negative showing that as the liquid is cooled, the molecules get closer and closer together as they solidify. 16. Which response includes all of the following compounds that exhibit hydrogen bonding and no other compounds? CH4, AsH5, CH3NH2, H2O, HF a) AsH5, H2O b) CH3NH2, HF c) CH4, AsH5, H2O d) AsH5, CH3NH2 e) HF, H2O, CH3NH2 f) HF, H2O g) HF, H2O, AsH5 h) none of the above 17. Twenty seven grams of a nonelectrolyte with a molecular weight of 220 g/mol is dissolved in 250.0 mL of benzene. Calculate the boiling point of the solution. The Kb for benzene = 2.53 oC/m. The boiling point of pure benzene is 80.1oC. The density of benzene = 0.876 g/mL. a) 80.1oC b) 78.7oC c) 80.2oC d) 79.4 oC e) 81.5oC f) 82.3oC g) 84.5oC h) 80.8oC 18. What is the mole fraction of methanol, CH3OH, in an aqueous solution that is 20.0% methanol by mass? a) 0.250 c) 0.101 e) 0.308 g) 0.218 b) 0.544 d) 0.123 f) 0.328 h) 0.378 19.(10 pts) For the following compound: _ | || _ H--N--C--C--O--H | | H H a) The bond angle between the hydrogens on the nitrogen is _______ b) The bond angle between the second carbon and hydrogen on the oxygen is _______ c) The bond angle between the oxygens on the second carbon is _______ d) The hybridization of atomic orbitals on the nitrogen is __________ e) The hybridization of atomic orbitals on the first carbon is __________ f) The hybridization of atomic orbitals on the second carbon is __________ 20. Estimate the enthalpy change for the following reaction using the given bond energies. (Be careful to get the correct structure of each compound first) C2H4(g) + H2O(g) ---> CH3CH2OH(g) C=C 602 kJ/mol C-O 358 kJ/mol a) -550. kJ b) -654 kJ C-H 413 kJ/mol C-C 346 kJ/mol c) 361 kJ d) -320 kJ e) -52 kJ f) 35 kJ O-H 463 kJ/mol g) -361 kJ h) 550 kJ








