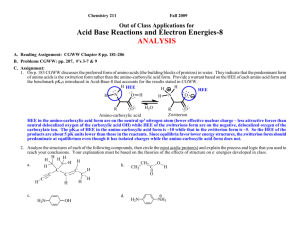
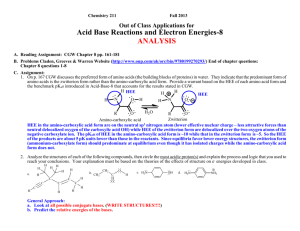
211-97 Acid-Base 94-6
advertisement

Chemistry 211 II. Fall 2008 Equilibrium Controlled Reactions Acid Base Reactions & Energy Relationships - 4 Out of Class Applications ANALYSIS : : : 1. For each of the following compounds, use the theory developed in Acid-Base-4 to select the most acidic proton(s): H H H H H H H N C C O H H H H C N C O C H H C C H H C H H H H H Then explain the process and logic that you used to reach your conclusions. H H H H H H H H loss of H H H C N C O C H H C N C O C H proton a H H a H Loss of proton a produces a new lone pair of e-'s on a nitrogen. Loss of any other proton in the molecule would lead to a new lone pair of e-'s on a carbon atom. Since the net charges are the same ( ) and nitrogen has a higher effective nuclear charge than carbon, the new e- pair on nitrogen should have lower energy than any of the e- pairs that might be formed on one of the carbon atoms due to increased e- - nuclear attraction caused by the higher nuclear charge in N vs. C. Consequently, there should be a smaller energy increase for loss of proton a than for any other proton in the molecule. So the loss of proton a should be the most favorable reaction and proton a should be the most acidic proton in the molecule. H cH H H Hb N C C O H H N C C O loss of a H H H C H C C H C H f proton a d C C H H H H H eH H H Loss of proton a produces a new lone pair of e-'s on a oxygen. Loss of proton b produces a new lone pair of e-'s on a nitrogen and loss of any other proton in the molecule (c-f) would lead to a new lone pair of e-'s on a carbon atom. Since the net charges are the same ( ) and oxygen has a higher effective nuclear charge than either nitrogen or carbon, the new e- pair on oxygen should have lower energy than any of the e- pairs that might be formed on any other atom due to increased e- - nuclear attraction caused by the higher nuclear charge in O vs. N or C. Consequently, proton a should be the most acidic proton in the molecule since its removal would require the smallest energy increase. : : : : : : : : : : : : : : : : : 2 Acid-Base Rxns & Electron Energy 2. Which of the following ions should have the lower energy. - : : : : - P H S: CH3 CH2 CH2 Explain your choice on the basis of the theory developed in this activity. The difference between the two ions is the substitution of phosphorus in the first ion for sulfur in the second ion. Consequently, the highest energy e-'s, the lone pair e-'s, are on different atoms in each ion. Since the net charges are the same, and the valence e-'s of sulfur and phosphorus are in the same principal energy level (both atoms are in the same row of the Periodic Table of the Elements) and sulfur, with more protons in its nucleus, should have a higher effective nuclear charge. So lone pair e-'s should have lower energy on the sulfur than on the phosphorus due to the higher electron-nuclear attraction for electrons on sulfur. Since all of the other e-'s in both ions are in similar bonds, the sulfur ion should have lower energy. CH3 CH2 CH2 3. For each of the following acid-base reactions, use the theory developed in this activity to predict whether the equilibrium constant should be > or < 1. Explain the logic that led you to your conclusion. a. H + O: + NH2 : NH : : O : : : : The fundamental change that occurs in this reaction is the conversion of an O-H bond plus a lone pair of e-'s on N in the reactants to an N-H bond plus a lone pair of e-'s on O . If we consider the HEE of the reactants and HEE on the products, we see the net change to be movement of HEE N O . Since the net charge of HEE’s is the same and O has a higher nuclear charge (stronger electron-nuclear attraction), then HEE are moving from a higher energy site (N ) to a lower energy site (O ) yielding a negative G˚ and an equilibrium constant > 1. : H + HEE NH :O HEE + NH2 : : : O : : : Acid-Base Rxns & Electron Energy b. O: - : + : + CH2 : : : CH3 3 O H H H The fundamental change that occurs in this reaction is the conversion of a C-H bond plus a lone pair of e-'s on O in the reactants to an O-H bond plus a lone pair of e-'s on C . If we consider the HEE of the reactants and HEE on the products, we see the net change to be movement of HEE O C . Since the net charge of HEE’s is the same and O has a higher nuclear charge (stronger electron-nuclear attraction), then HEE are moving from a lower energy site (O ) to a higher energy site (C ) yielding a positive G˚ and an equilibrium constant < 1. + HEE CH2 : + O: H : : CH3 : HEE: O H H 4. For the following pair of equilibrium controlled reactions, predict which one should produce more product at equilibrium. Explain your choice on the basis of the theory developed in this activity. -S O + + O S -S NH - - HN S The difference in these two reactions is the substitution of a nitrogen atom in the second reaction for an oxygen atom in the first. In the reactions, a lone pair of e-'s on S (HEE of reactants in both reactions) is used to form a C-S bond and a new lone pair of e-'s is formed on another atom. The new lone pair of e-'s forms on an O , HEE of the products of the first reaction, and on an N , HEE of the products of the second reaction. Thus the HEE of e-'s in the reactants are on an S in both reactions but on different atoms in the products of each. Thus we can judge the reactants of both reactions to have about equally energy while their products are different. The more favorable reaction in this problem should be the one with the lower energy products yielding a lower G˚. Oxygen has a higher effective nuclear charge than does nitrogen so there should be stronger electron-nuclear attraction on HEE in the products of the first reaction than the corresponding HEE in the second. Since the HEE are considerably lower in energy in the products of the first reaction than in the second reaction and HEE of both reactants have the same energy, the first reaction should have a lower G˚ and be more favorable than the second.