pola27477-sup-0001-suppinfo
advertisement

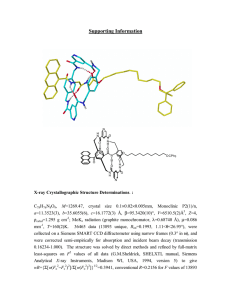
Supporting Information: Blue LED Light-Sensitive Benzo Pyrazolo (or Imidazo) Isoquinolinone Derivatives in High Performance Photoinitiating Systems for Polymerization Reactions. Pu Xiao,a Frédéric Dumur,b Bernadette Graff,a Jing Zhang,a Fabrice Morlet-Savary,a Didier Gigmes,b Jean Pierre Fouassier1 and Jacques Lalevée*,a a Institut de Science des Matériaux de Mulhouse IS2M, UMR CNRS 7361, ENSCMu-UHA, 15, rue Jean Starcky, 68057 Mulhouse Cedex, France. b Aix-Marseille Université, CNRS, ICR, UMR 7273, F-13397 Marseille, France. Corresponding Authors: jacques.lalevee@uha.fr All reagents and solvents were purchased from Aldrich or Alfa Aesar and used as received without further purification. Mass spectroscopy was performed by the Spectropole of Aix-Marseille University. ESI mass spectral analyses were recorded with a 3200 QTRAP (Applied Biosystems SCIEX) mass spectrometer. The HRMS mass spectral analysis was performed with a QStar Elite (Applied Biosystems SCIEX) mass spectrometer. Elemental analyses were recorded with a Thermo Finnigan EA 1112 elemental analysis apparatus driven by the Eager 300 software. 1H and 13C NMR spectra were determined at room temperature in 5 mm o.d. tubes on a Bruker Avance 400 spectrometer of the Spectropole: 1H (400 MHz) and 13 C (100 MHz). The 1H chemical shifts were referenced to the solvent peak CDCl3 (7.26 ppm), DMSO (2.49 ppm) and the 13C chemical shifts were referenced to the solvent peak CDCl3 (77 ppm), DMSO (49.5 ppm). All these dyes were prepared with analytical purity up to accepted 1 Formerly, ENSCMu-UHA, 3 rue Alfred Werner, 68093 Mulhouse Cedex, France. standards for new organic compounds (>98%) which was checked by high field NMR analysis. For IQ1 and IQ3, the mixture of isomers has been separated for identification. Synthesis of 3-(hexylamino)-7H-benzo[de]benzo[4,5]imidazo[2,1-a]isoquinolin-7-one IQ1 and 4-(hexylamino)-7H-benzo[de]benzo[4,5]imidazo[2,1-a]isoquinolin-7-one IQ2 To a stirred solution of 3-bromo-7H-benzo[de]benzo[4,5]imidazo[2,1-a]isoquinolin-7-one IQ3 (1.74 g, 5.00 mmol) in DMF (15 mL) was added hexylamine (1.97 mL, 1.52 g, 15.00 mmol). The mixture was then refluxed overnight. After completion, the reaction mixture was cooled to room temperature and concentrated under vacuum until most of the solvent was removed. Addition of pentane precipitated a solid identified as one of the two initial isomers (IQ1) (0.52 g, 28% yield). The residue was purified by column chromatography (SiO2) using DCM as the eluent. After evaporation of the volatiles, addition of acetonitrile precipitated an orange solid which was filtered off and dried under vacuum. The second precipitate was identified as being the second isomer IQ2 (0.63 g, 34 % yield). IQ1 : 1H NMR (CDCl3) δ (ppm): 0.87-0.92 (m, 6H), 1.28-1.41 (m, 12H), 1.47-1.55 (m, 2H), 1.63-1.66 (m, 2H), 3.28 (t, 2H, J = 6.6 Hz, NH-CH2), 3.30 (t, 2H, J = 6.6 Hz, NH-CH2), 5.345.37 (m, 2H), 6.47 (d, 1H, J = 8.4 Hz), 7.00 (d, 1H, J = 8.2 Hz), 7.44-7.48 (m, 5H), 7.59 (t, 1H, J = 7.8 Hz), 7.76 (d, 1H, J = Hz), 7.84 (d, 2H, J = 8.2 Hz), 8.17 (s, 1H), 8.24 (d, 1H, J = 8.3 Hz), 8.35 (d, 1H, J = 8.5 Hz), 8.49-8.53 (m, 4H), 8.60 (d, 1H, J = 7.0 Hz), 8.69 (d, 1H, J = 7.1 Hz); 13 C NMR (CDCl3) δ (ppm): 13.93, 13.98, 22.46, 22.53, 26.44, 26.76, 28.8, 29.4, 31.3, 31.5, 38.1, 43.6, 44.7, 104.3, 109.8, 113.0, 114.5, 115.8, 115.9, 119.4, 119.5, 120.1, 120.3, 120.5, 123.2, 123.4, 124.7, 124.9, 125.0, 125.23, 125.25, 126.8, 129.0, 131.8, 131.9, 133.1, 134.8, 143.6, 143.7, 149.5, 149.6, 150.2, 157.8, 160.4, 160.5, 161.1; HRMS (ESI MS) m/z: theor: 369.1841 found: 369.1844 (M+. detected). IQ2 : 1H NMR (CDCl3) δ (ppm): 0.93 (t, 3H, J = 7.1 Hz), 1.36-1.38 (m, 6H), 1.71-1.74 (m, 4H), 3.23-3.28 (m, 2H), 5.37 (t, 1H, J = 6.5 Hz), 6.61 (d, 1H, J = 8.5 Hz), 7.44-7.46 (m, 2H), 7.57 (t, 1H, J = 7.4 Hz), 7.86 (d, 1H, J = 7.4 Hz), 8.49 (d, 1H, J = 8.5 Hz), 8.56-8.59 (m, 1H), 8.72 (d, 1H, J = 7.3 Hz); 13C NMR (CDCl3) δ (ppm): 14.0, 22.6, 26.8, 28.9, 31.5, 43.7, 104.5, 110.2, 116.0, 119.5, 120.3, 120.7, 123.3, 124.6, 125.0, 125.1, 127.0, 128.5, 132.0, 135.1, 143.7, 149.6, 150.2, 160.6; HRMS (ESI MS) m/z: theor: 369.1841 found: 369.1839 (M+. detected). Synthesis of 3-bromo-7H-benzo[de]benzo[4,5]imidazo[2,1-a]isoquinolin-7-one IQ3 6-Bromobenzo[de]isochromene-1,3-dione (1.85 g, 6.68 mmol) and o-phenylenediamine (0.72 g, 6.68 mmol) was suspended in acetic acid (50 mL) and the solution was refluxed overnight. During reflux, a yellow precipitate formed. After cooling, the solvent was removed under reduced pressure. The residue was suspended in pentane, washed several times with pentane and dried under vacuum. The product was obtained under the form of a mixture of isomers in nearly quantitative yield. After several recrystallization in a mixture of DMF/toluene, the title molecule could be isolated pure (0.82 g, 35% yield). 1H NMR (CDCl3) δ (ppm): 7.48-7.50 (m, 2H), 7.86-7.89 (m, 1H), 7.92 (d, 1H, J = 7.9 Hz), 8.08 (d, 1H, J = 7.9 Hz), 8.52-8.54 (m, 1H), 8.65 (t, 1H, J = 8.3 Hz), 8.53 (d, 1H, J = 7.2 Hz); 13 C NMR (CDCl3) δ (ppm): 115.8, 120.0, 120.3, 123.6, 125.7, 126.0, 127.0, 127.5, 128.0, 128.1, 131.2, 131.5, 131.8, 132.4, 134.5, 143.8, 148.6, 160.0; 1H NMR (DMSO d6) δ (ppm): 7.51-7.53 (m, 2H), 7.90-7.91 (m, 1H), 8.10 (t, 1H, J = 7.9 Hz), 8.28 (d, 1H, J = 7.8 Hz), 8.43 (d, 1H, J = 7.9 Hz), 8.62 (d, 1H, J = 8.4 Hz), 8.67 (d, 1H, J = 8.4 Hz), 8.80 (d, 1H, J = 7.2 Hz); HRMS (ESI MS) m/z: theor: 348.9971 found: 348.9975 ((M+H)+ detected). Figure S1. The emission spectrum of the halogen lamp. 1,0 0,8 I (a.u.) 0,6 0,4 0,2 0,0 380 400 420 440 460 480 500 (nm) Figure S2. The emission spectrum of the blue LED centered at 477 nm. 200 160 2 I (A/cm ) 120 80 40 0 -40 0.0 0.5 1.0 1.5 2.0 E (V) Figure S3. Cyclic voltammogram of IQ1 (measured in acetonitrile with tetrabutylammonium hexafluorophosphate as a supporting electrolyte).