Problem 3 - Chapter 20 problem 72 m N (v)
advertisement

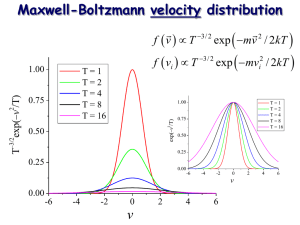
Problem 3 - Chapter 20 problem 72 The number distribution for molecules in an ideal gas is N (v) v = 4 N m 2 kT 3=2 v2e where A0 = 4 mv 2 =2kT m 2 kT v = N A0 v 2 e mv 2 =2kT v 3=2 ; hence the probability distribution is P (v) v = N (v) v = A0 v 2 e N mv 2 =2kT v: (a) The most probable molecular speed is found at the maximum of this probability distribution. Taking the derivative of this distribution and setting it equal to zero, we …nd d P (vo ) = A0 2vo dv kT vo2 = 2 : m vo3 m=kT e mv 2 =2kT = 0; Since the rms velocity is given by 2 v 2 = vrms =3 kT ; m we see that the most probable speed is related to the rms velocity via vo = (2=3)1=2 vrms = :816vrms (b) The average velocity is Z Z 1 1 1 hvi = vN (v) dv = A0 v3e N 0 0 mv 2 =2kT Integrating by parts by de…ning x = v 2 and dy = ve dx = 2vdv and y = 1 kT e m dv: mv 2 =2kT mv 2 =2kT : we …nd Hence 2 kT mv 2 =2kT hvi = A0 v hvi = 2kT kT A0 e m m m 1=2 hvi = e 1 0 1 mv 2 =2kT (kT ) 161=2 = m1=2 (2 )1=2 r 2kT + A0 m =2 0 Z kT m 1 ve mv 2 =2kT dv 0 2 A0 = 2 kT m 2 4 m 2 kT 3=2 8 kT m From this the relation between the average speed and the rms velocity is r 8 hvi = vrms = :921vrms 3 (c) Note from the kinetic theory of an ideal gas the rms velocity is v 2 = 3kT =m; where m is the mass of an individual molecule. From the ideal gas law P V = N kT so that P = N kT =V = M kT N m kT = = kT =m; V m V m where M is the total mass of the gas. The speed of sound is given by vs2 = P = kT =m vs = ( =3)1=2 vrms : Since 5=3 for a monatomic gas and 7=5 for a diatomic gas, we see that the speed of sound is also similar to the rms velocity of a molecule. Problem 4 - Chapter 21 problem 66 (a) The bulk modulus is de…ned as P : V =V B= When the bulk modulus is pressure dependent then a more general expression is the derivative form @P B= V : @V 2 For an adiabatic expansion (expansion and compression in a sound wave is adiabatic) then P V = const: Di¤erentiating this expression with respect to the volume yields @P V + PV @V 1 =0! @P = @V P : V Substituting this result into the expression for the bulk modulus yields B = P: Thus the speed of sound is vs = p B= = p P= : (b) Since the mass density, ; of a gas (or any material) depends on it mass divided the volume, = m=V; the bulk modulus can be expressed via the chain rule as B = B = m @P @ = @ @V @P : @ m @P @ m V2 The speed of sound can then be written as s p @P ; vs = B= = @ which is the desired result. 3 2 = @P @