Supplementary materials: Effects of aeration and internal recycle
advertisement

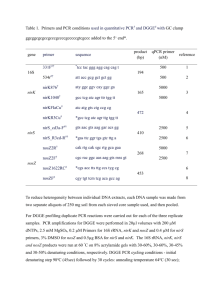
Supplementary materials: Effects of aeration and internal recycle flow on nitrous oxide emission from a Modified Ludzak–Ettinger process fed with glycerol Kang Song1,2, Toshikazu Suenaga1, Willie F. Harper Jr.3, Tomoyuki Hori4, Shohei Riya1, Masaaki Hosomi1, Akihiko Terada1* 1. Department of Chemical Engineering, Tokyo University of Agriculture & Technology, Tokyo 184-8588, Japan 2. Research Fellow of Japan Society for the Promotion of Science, 5-3-1 Kojimachi, Chiyoda-ku, Tokyo 102-0083, Japan 3. Department of Systems Engineering and Management, Air Force Institute of Technology, Wright-Patterson Air Force Base, OH 45433, United States 4. National Institute of Advanced Industrial Science and Technology, Tokyo 305-8568, Japan * Corresponding author: akte@cc.tuat.ac.jp Tel: +81-42-388-7069 / Fax: +81-42-388-7731 I Figure legends for supplementary materials Fig. S1 Time courses of dissolved N2O concentration in the anoxic and aerobic tanks on day 146. Fig. S2 Representative organic carbon and nitrogen concentrations in the MLE under different IRFs conditions. The samples were taken at the final date of the IRF condition (prior to changeover). Fig. S3 Time courses of the quantities of functional genes encoding (a) amoA, (b) nirK, (c) nirS, (d) nosZ clade I, and (e) nosZ clade II. Fig. S4 Correlation of N2O emission rate from the MLE system versus the relative gene abundance nosZ/(nirK + nirS). Fig. S5 Correlation of N2O emission rate from the MLE system versus relative abundance of the genus Rhodobacter. Fig. S6 Relative abundances of AOB and NOB during the MLE operation. II 70 60 50 40 30 20 10 0 13:18 13:32 13:46 14:01 14:15 14:29 14:44 14:58 15:12 15:27 15:41 15:55 16:10 16:24 16:38 16:53 17:07 17:21 17:36 17:50 18:04 18:19 18:33 18:47 19:02 19:16 19:30 19:45 19:59 20:13 20:28 20:42 20:57 21:11 21:25 21:40 21:54 22:08 22:23 22:37 22:51 Dissolved N2O concentration (μgN/L) 80 Time of operation (Hour:minute) on day 146 Anoxic tank Aerobic tank 350 Nitrogen concentration (mgN/L) 70 IRF = 3.6 IRF = 7.2 IRF = 1.0 60 300 50 250 40 200 30 150 20 100 10 50 0 0 NH4+ NO2- NO3- Total organic carbon concentration (mgN/L) Fig. S1 Time courses of dissolved N2O concentration in the anoxic and aerobic tanks on day 146. TOC Fig. S2 Representative organic carbon and nitrogen concentrations in the MLE under different IRF conditions. The samples were taken at the final date of each IRF condition (prior to changeover). III Quantity (copy/ng-DNA) (a). amoA 1.E+04 Low aeration High aeration 1.E+03 1.E+02 1.E+01 1.E+00 106 113 131 148 194 203 228 (b). Quantity (copy/ng-DNA) Day of operation 1.E+07 1.E+06 nirK Low aeration High aeration 1.E+05 1.E+04 1.E+03 1.E+02 1.E+01 1.E+00 106 113 131 148 194 203 228 (c). Quantity (copy/ng-DNA) Day of operation 1.E+07 1.E+06 nirS High aeration Low aeration 1.E+05 1.E+04 1.E+03 1.E+02 1.E+01 1.E+00 106 113 131 148 194 Day of operation IV 203 228 Quantity (copy/ng-DNA) (d). nosZ clade I 1.E+07 High aeration Low aeration 1.E+06 1.E+05 1.E+04 1.E+03 1.E+02 1.E+01 1.E+00 106 113 131 148 194 203 228 Day of operation (e). Quantity (copy/ng-DNA) nosZ clade II 1.E+05 High aeration Low aeration 1.E+04 1.E+03 1.E+02 1.E+01 1.E+00 106 113 131 148 194 203 228 Day of operation Fig. S3 Time courses of the quantities of functional genes encoding (a) amoA, (b) nirK, (c) N2O emission rate (μgN/min) nirS, (d) nosZ clade I, and (e) nosZ clade II. 4 3 R² = 0.7499 P=0.012<0.05 2 1 0 0 1 2 nosZ/(nirS+nirK) 3 Fig. S4 Correlation of N2O emission rate from the MLE system versus the relative gene abundance nosZ/(nirK + nirS). V Percentage of Rhodobacter (%) 20 R² = 0.5848 P = 0.045 < 0.05 15 10 5 0 0 1 2 3 N2O emission rate (μgN/min) 4 Fig. S5 Correlation of N2O emission rate from the MLE system versus relative abundance of the genus Rhodobacter. High aeration Low aeration Fig. S6 Relative abundances of AOB and NOB during the MLE operation VI Table S1 Primers used in real-time quantitative PCR Primer Purpose Target gene Sequence (5’-3’) Reference amoA1F qPCR amoA GGGGTTTCTACTGGTGGT (Rotthauwe et al. 1997) amoA 2R qPCR amoA CCCCTCKGSAAAGCCTTCTTC nirk 876-F qPCR nirK ATYGGCGGVCAYGGCGA (Henry et al. 2004) nirK 1040-R qPCR nirK GCCTCGATCAGRTTRTGGTT nirS 4QF qPCR nirS GTSAACGYSAAGGARACSGG (Throback et al. 2004) nirS 6QR qPCR nirS GASTTCGGRTGSGTCTTSAYGAA nosZ1F qPCR nosZ WCSYTGTTCMTCGACAGCCAG (Henry et al. 2006) nosZ1R qPCR nosZ ATGTCGATCARCTGVKCRTTYTC nosZ Clade II-F qPCR nosZ CTI GGICCI YTK CAY AC (Jones et al. 2013) nosZ Clade II-R qPCR nosZ GCI GARCAR AAI TCB GTR C Table S2 Diversity indices of microbial community structure Glycerol Read number 0 98 106 113 131 148 154 167 194 32196 31336 35856 25540 38560 37108 46564 35033 36846 OTUs number (<97%) 1123 1002 1128 1197 1136 1345 1350 993 1122 Shannon–Wiener’s H’ 41.06 35.74 18.84 25.20 20.03 26.11 19.55 30.43 22.99 Evenness J 5.85 5.17 2.68 3.56 2.85 3.62 2.71 4.41 3.27 VII Additional References Henry S, Baudoin E, López-Gutiérrez JC, Martin-Laurent F, Brauman A, Philippot L (2004) Quantification of denitrifying bacteria in soils by nirK gene targeted real-time PCR. Journal of microbiological methods 59:327-335 Henry S, Bru D, Stres B, Hallet S, Philippot L (2006) Quantitative detection of the nosZ gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils. Appl Environ Microbiol 72:5181-9 Jones CM, Graf DR, Bru D, Philippot L, Hallin S (2013) The unaccounted yet abundant nitrous oxide-reducing microbial community: a potential nitrous oxide sink. The ISME journal 7:417-26 Rotthauwe J-H, Witzel K-P, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Applied and environmental microbiology 63:4704-4712 Throback IN, Enwall K, Jarvis A, Hallin S (2004) Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS microbiology ecology 49:401-17 VIII