Therapeutic Drug Monitoring and Pharmacokinetics
advertisement

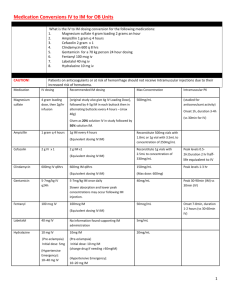
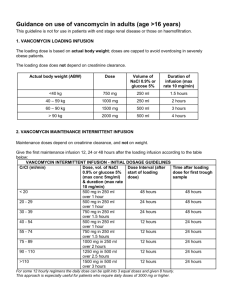
Approved by P+T, 11/04 Therapeutic Drug Monitoring and Pharmacokinetics of Intravenous Vancomycin for Pharmacists and Other Healthcare Professionals Purpose of this document: This document is a Section of Infectious Disease & Pharmacy consensus statement of agreed upon dosing and drug monitoring recommendations. Comprehensive Antimicrobial Program (CAP) Approved Indications: Note that vancomycin is a class III agent that requires CAP review within 72 hours Prophylactic use: 1. Peri-operative prophylaxis in patients with known severe allergy to ßlactams. Empiric use: 1. Pneumococcal meningitis. 2. Neonatal sepsis. 3. Infection of a prosthesis. 4. Life-threatening presumed or documented S. aureus infection, pending sensitivities. 5. Patients with neutropenic fever if line infection thought likely (e.g., signs of inflammation over catheter site). General 1. Treatment of documented methicillin-resistant Staphylococcus aureus (MRSA) infections 2. Treatment of a documented ampicillin resistant (vancomycin sensitive) enterococcal infection 3. Treatment of enterococcus in ampicillin-allergic patients Vancomycin is a glycopeptide antibiotic that has activity against a wide variety of gram positive aerobic bacteria, including resistant organism such as methicillin resistant Staphylococcus aureus (MRSA) penicillin-resistant Streptococcus pneumoniae and sensitive Enterococcus species. Dosing I. Adult Empiric dosing A. Calculate Creatinine Clearance (CrCL): (ml/min) a. Estimated CRCL using Crockoft and Gault method1: 140-age x IBW x 0.85 (females only) 72 x Serum creatinine (mg/dl) B. Determine dosing weight (DW) a. Dosing weight should be actual body weight2 1 Approved by P+T, 11/04 B. Use urine CrCL if available: Urine volume (ml/24hrs) 1440 x x Urine creatinine (ml/dl) Serum creatinine (mg/dl) C. Modified Matzeke Nomogram Use chart below to determine the dose and interval (round calculation to the nearest 250mg dose) CrCL (ml/min) Dose (mg/Kg) Dosing interval (hrs) 120-81 15 (use 1gm q12h for most pts who weigh 55100Kg) Q12H 80-61 60-41 40-31 30-16 <15 22 15 22 15 15 Q24H Q24H Q48H Q48H x1 then check level in 2 days, re-dose when level is 10-15mg/L Hemodialysis 15 x1 then check level in 5 days, re-dose when level is 10-15mg/L (usually Q6 days) D. Neonates Weight <1.2 Kg 1.2-2 Kg >2 Kg Prenatal age < 7 days 15mg/Kg/dose Q24H 10-15mg/Kg/dose Q12-18H 10-15mg/Kg/dose Q8-12H Postnatal age > 7 15mg/Kg/dose Q24H 10-15mg/Kg/dose Q8-12H 15-20mg/Kg/dose Q8H *All Pediatric dosing based on The Harriet Lane Handbook, Sixth edition II. III. E. Infants and Children a. 40mg/Kg/day divided Q6H or Q8H b. Max of 1Gm/dose Morbidly obese patients (>90% over ideal body weight) A. With normal renal function a. Dose=30mg/Kg/Day divided Q8H D. Renal insufficiency a. Use Modified Matzeke Nomogram for dose and dosing interval Dosage adjustments A. Adult patients not requiring therapeutic drug levels (see below) a. Dose =15mg/Kg b. Calculate CrCL and choose appropriate dosing interval based on the Modified Matzke Nomogram B. Patients requiring a trough level only (see below) 2 Approved by P+T, 11/04 C. a. Trough <5mg/LIncrease dosing frequency to the next standard dosing interval (e.g. q12h to q8h) i. Standard dosing intervals are defined as (Q6H, Q8H, Q12H, Q24H, Q48H) b. Trough 5-20mg/LNo change c. Trough >15mg/L Decrease dose by 50% d. If a change is made check a trough level 3-5 half-lives later Patients requiring a peak and trough level use the Sawchuck-Zaske method4 (see below). PK Dose-Pro can be used as a double check of your calculations or if you feel comfortable with the equations you may use PK Dose-Pro and double check the results a. Generally peak levels between 20 and 40mcg/ml are considered acceptable b. Generally trough levels between 5 and 15mcg/ml are considered acceptable Volume of Distribution (Vd) o Normal range=0.5-0.9L/Kg o Average=0.7L/Kg Elimination Rate Constant (Ke) o Good estimate is 0.00083(CRCL)+0.0044 1st find Ke Ke = lnC1 t1-t2 lnC2___ 2nd find T1/2 (Normally want dosing interval to be 2-3 X T1/2) T1/2= 0.693 Ke 3rd find true peak (Cmax) and trough (Cmin) Cmax= __C1_____ e-Ke(t”) Cmin= Cmax (e-Ke(T-t’) ) 4th find Vd in liters Vd= Dose (mg) x (1-e-Ke(t’) ) t’*Ke (Cmax – Cmin e-Ke(t’) ) Symbol Meaning (D) (t’) Dose (mg) Infusion duration (hr) Dosing interval (hr) Time since infusion completion (hr) Time since completion of infusion when C1 drawn (hr) Volume of distribution (L) (T) (t) (t”) (Vd) 5th Find New Dose (mg) Dose= Cmax (desired) * Ke*Vd*(1-e-Ke(T) )*t’ 3 Approved by P+T, 11/04 1-e-Ke(t’) 6th Calculate new peak and trough Cmax(new)= _Dose(mg)* (1-e-Ke(t’) )_ t’*Ke*Vd (1-e-ke(T) ) Cmin(new)= Cmax (e-Ke(T-t’) ) 7th If a change is made check a peak and trough level 3-5 half-lives later Patient specific values requiring monitoring I. Therapeutic drug levels A. Most patients DO NOT require serum levels B. Patients NOT requiring therapeutic drug levels a. Adult patients with the following features: i. <60y/o + Normal body weight + Stable renal function + CrCL >30ml/min or anticipated treatment course < 3 days II. Patients that may require a TROUGH LEVEL ONLY A. Renal failure (CrCL<30ml/min)5 B. Adult patients with altered volumes of distribution or renal clearance a. Burn patients6 b. Critically ill8 c. Extremes in body weight i. Obese (>90% of IBW)3 ii. Adults weighing <50kg5 C. Neutropenia9 D. Concomitant nephrotoxins a. Aminoglycosides5, 9-12 b. Amphotericin B5,9 c. IV contrast dye5 d. Other medications5: cyclosporine, tacrolimus, III. Patients requiring both PEAK AND TROUGH LEVELS A. Higher doses are needed to penetrate the sight of infection or treatment of serious / life threatening infections a. Bacterial meningitis B. Patients receiving CVVHD or PD C. Pediatric patients less than 6 years of age due to developing / changing vancomycin pharmacokinetics IV. Sampling times A. Trough levels should be obtained within 30 minutes of the next dose B. Peak levels should be drawn 1 hour after the completion of infusion b. Levels should be obtained at steady state concentrations i. Steady state concentrations are achieved after 3-5 half lives 4 Approved by P+T, 11/04 1. Usually around the third or fourth maintenance dose. c. Levels should be scheduled during the day if possible to ensure follow-up d. Infusion time i. Two hours is the preferred infusion duration and should be considered the standard ii. The maximum infusion time should be 60 minutes per 1 gram of vancomycin administered V. Therapeutic range based on current DHMC laboratory guidelines A. Optimal peak and trough levels have not been established in clinical studies B. Peak 20-40 mg/L C. Trough 5-10mg /L D. Higher peak and trough levels may be considered in patients with bacterial meningitis VI. Laboratory parameters A. Serum creatinine a. Monitor for trends and adjust doses based on nomogram (CrCL) if not following serum drug levels B. Blood urea nitrogen a. BUN / Scr ratio of > 20 may indicate a pre-renal state (dehydration) representing a smaller volume of distribution (VD) and subsequent increased levels C. White blood cell count (WBC) a. Trends in WBC count help evaluate physiologic response to treatment D. Microbiology a. Gram stain results i. Intravenous vancomycin is effective against gram positive cocci (GPC) only b. Culture and sensitivity results i. Culture 1. These results should be reviewed to identify the species of the microorganism. ii. Sensitivities 1. Identifies in vitro activity of antibiotics against a specific microorganism VII. Monitor for Adverse Drug Reactions (ADR) A. Red-man syndrome (erythematous flushing of the face, neck and upper extremities) a. Usually related to histamine release in response to a rapid infusion. b. Up to 47% of patients receiving vancomycin experience this ADR 5 Approved by P+T, 11/04 c. Recommended course of action is to double the infusion period B. Nephrotoxicity a. Rare when vancomycin is used alone (<5% of patients) b. The concurrent use of aminoglycosides or other nephrotoxins seems to enhance the risk of nephrotoxicity c. Recommended course of action is to withdraw one of the offending agents and risk/benefit should be discussed with the physician C. Ototoxicity a. A rare occurrence b. More commonly reported in conjunction with impaired renal function, persistent high serum concentrations(~80mg/L) and concomitant use of aminoglycosides c. Recommended course of action is to withdraw the offending agent and risk/benefit should be discussed with the physician D. Trombophlebitis a. Occurs in roughly 13% of patients with peripheral catheters b. Recommended course of action: thrombophlebitis will usually settle with rest, elevation of the limb, and (if necessary) antiinflammatory drugs E. Reversible Neutropenia a. Up to 2% of Vancomycin treated patients experience this ADR b. Recommended course of action is to withdraw the offending agent risk/benefit should be discussed with the physician VIII. Other information A. Vital signs a. Fever curve b. Blood pressure c. Respiratory rate d. Heart rate B. Fluid status (I/O) 6 Approved by P+T, 11/04 References 1. Cockroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976:16;31-41. 2. Matzeke GR, et al. Antimicrob Agent Chemother 1984;25(4):433-7 3. Bauer LA, et al. Vancomycin dosing in morbidly obese patients. Eur J. Clin Pharmacol 1998:54;621-625. 4. Sawchuk RJ, Zaske DE, et al. Kinetic model for gentamicin dosing. Clin Pharmacol Ther 1977;21;3:362-369. 5. Karam CM, et al. Outcome assessment of minimizing vancomycin monitoring and dosing adjustments. Pharmacotherapy 1999:19;257-66 6. Rybak MJ, et al. Vancomycin pharmacokinetics in burn patients and intravenous drug abusers. Antimicrob Agent Chemother 1990:34;792-5. 7. Cutler NR, et al. Vancomycin disposition: the importance of age. Clin Pharmacol Ther. 1984 Dec;36:803-10. 8. Garaud JJ, et al. Vancomycin pharmacokinetics in critically ill patients. J Antimicrob Chemother. 1984 Dec;14 Suppl D:53-7. 9. Pauly DJ, Musa DM, Lestico MR, Lindstrom MJ, Hetsko CM. Risk of nephrotoxicity with combination vancomycin-aminoglycoside antibiotic therapy. Pharmacotherapy 1990;10:378-82 10. Farber BF, Moellering RC. Retrospective study of the toxicity of preparations of vancomycin from 1974 to 1981. Antimicrob Agents Chemother 1983;23:138-41. 11. Rybak MJ, Albrecht LM, Boike SC, Chandrasekar PH. Nephrotoxicity of vancomycin, alone and with an aminoglycoside. J Antimicrob Chemother 1990;25:679-87. 12. Cimino MA, Rotstein C, Slaughter RL, Emrich LJ. Relationship of serum antibiotic concentrations to nephrotoxicity in cancer patients receiving concurrent aminoglycoside and vancomycin therapy. Am J Med 1987;83:1091-7. 7