Assg 10
advertisement

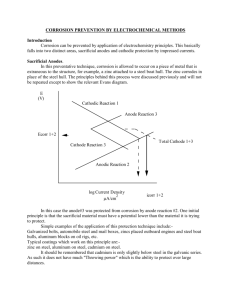
Assignment #10 To be completed by. Tues, Oct. 29, 2002 Jones, Chapter 6: 6.2 6.4 Assignment #11 To be turned in Tues., Nov. 5, 2002 The homework problems below (from the text) will be graded in lieu of a quiz on Nov. 5. 6.7 answers below: (a) icorr for 1cm2 of A = 2 x 10-3 A/cm2, (b) icorr for 10 cm2 of A = 1 x 10-2 A/cm2, (c) by trial and error icorr for A is 2.95 x 10-5 A/cm2 with a potential difference between A and C of about 0.28 V for R = 3000 . 6.10 (corrected) Determine the galvanic corrosion rate in aerated 3% NaCl of the anode in a couple between equal areas of (a) carbon steel (iron) and 316 stainless steel and (b) between equal areas of 316 stainless steel and titanium (c) show the effect of a 15:1 ratio of cathode to anode in (a) (d) explain the difference, if any, between the galvanic corrosion rates in (a) and (b). Graduate students only: 6.12 A difference in dissolved oxygen concentration (oxygen concentration cell) can often lead to corrosion at specific sites while other sites on the same surface do not corrode. Answer the following questions for an oxygen concentration cell: (a) Draw a sketch of an oxygen concentration cell on a steel surface, specifying the locations of the anodic and cathodic reactions. ALSO, write down the anodic and cathodic half cell reactions that occur at each of the two locations. (b) Draw the polarization diagram for this case using Figure 6.24 as a guide. Assume equal anodic and cathodic areas. Indicate on your sketch: Ecorr for the cathode, Ecorr for the anode, the Ecouple and the icouple. Note: Figure 6.24 includes two polarization curves for the reduction process, one for the oxygen rich site and a slightly different one for the oxygen starved site. (c) It is observed that the electrolyte near the oxygen starved site becomes more acidic with time. Explain how this can happen.