Aquatic Biomes: Salinity/Density & Temperature
advertisement
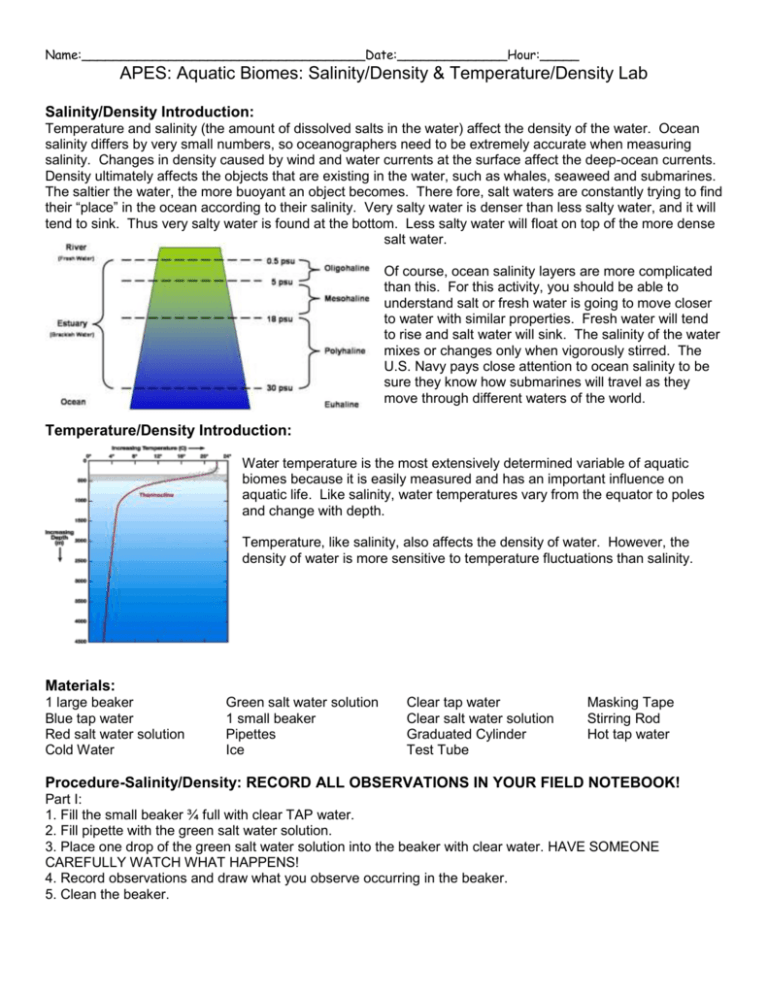
Name:____________________________________Date:______________Hour:_____ APES: Aquatic Biomes: Salinity/Density & Temperature/Density Lab Salinity/Density Introduction: Temperature and salinity (the amount of dissolved salts in the water) affect the density of the water. Ocean salinity differs by very small numbers, so oceanographers need to be extremely accurate when measuring salinity. Changes in density caused by wind and water currents at the surface affect the deep-ocean currents. Density ultimately affects the objects that are existing in the water, such as whales, seaweed and submarines. The saltier the water, the more buoyant an object becomes. There fore, salt waters are constantly trying to find their “place” in the ocean according to their salinity. Very salty water is denser than less salty water, and it will tend to sink. Thus very salty water is found at the bottom. Less salty water will float on top of the more dense salt water. Of course, ocean salinity layers are more complicated than this. For this activity, you should be able to understand salt or fresh water is going to move closer to water with similar properties. Fresh water will tend to rise and salt water will sink. The salinity of the water mixes or changes only when vigorously stirred. The U.S. Navy pays close attention to ocean salinity to be sure they know how submarines will travel as they move through different waters of the world. Temperature/Density Introduction: Water temperature is the most extensively determined variable of aquatic biomes because it is easily measured and has an important influence on aquatic life. Like salinity, water temperatures vary from the equator to poles and change with depth. Temperature, like salinity, also affects the density of water. However, the density of water is more sensitive to temperature fluctuations than salinity. Materials: 1 large beaker Blue tap water Red salt water solution Cold Water Green salt water solution 1 small beaker Pipettes Ice Clear tap water Clear salt water solution Graduated Cylinder Test Tube Masking Tape Stirring Rod Hot tap water Procedure-Salinity/Density: RECORD ALL OBSERVATIONS IN YOUR FIELD NOTEBOOK! Part I: 1. Fill the small beaker ¾ full with clear TAP water. 2. Fill pipette with the green salt water solution. 3. Place one drop of the green salt water solution into the beaker with clear water. HAVE SOMEONE CAREFULLY WATCH WHAT HAPPENS! 4. Record observations and draw what you observe occurring in the beaker. 5. Clean the beaker. Name:____________________________________Date:______________Hour:_____ Part II: 1. Fill the clean small beaker ¾ full with clear SALT water. 2. Fill the other pipette with blue tap water. 3. Place one drop of blue tap water into the beaker with the clear salt water solution. HAVE SOMEONE CAREFULLY WATCH WHAT HAPPENS! 4. Record observations and draw what you observe occurring in the beaker. 5. Clean the beaker. Part III: 1. Fill the large beaker with 100 ml of the green salt water solution. 2. Pour 50 ml of clear tap water slowly into the beaker on top of the green salt water solution. HAVE SOMEONE CAREFULLY WATCH WHAT HAPPENS! (Hint: After a point in time, the two layers should not mix). 3. Record observations. 4. Fill a new pipette with the red salt water solution. 5. Place the pipette into the green salt water layer (near the bottom) and squeeze out a drop of the red salt water solution. HAVE SOMEONE CAREFULLY WATCH WHAT HAPPENS! 6. Record observations and draw what you observe occurring in the beaker. 7. Take the same pipette with the red salt water solution and place it into the clear tap water layer (near the top). Squeeze out a drop of the red salt water solution. HAVE SOMEONE CAREFULLY WATCH WHAT HAPPENS! 8. Record observations and draw what you observe occurring in the beaker. 9. Using a stirring rod, mix the layered water system together. HAVE SOMEONE CAREFULLY WATCH WHAT HAPPENS! 10. Record observations and draw what you observe occurring in the beaker. Procedure-Temperature/Density: RECORD ALL OBSERVATIONS IN YOUR FIELD NOTEBOOK! 1. Fill a 100 ml graduated cylinder with COLD water to the 50 ml mark. 2. Put 2-3 drops of food coloring in a test tube and fill it half full with HOT water. 3. Pour the contents of the test tube slowly into the cylinder and record your observations. 4. Empty the 100 ml graduated cylinder and refill it with HOT water. 5. Add a test tube full of COLD water and 2-3 drops of food coloring to some ice in a beaker. Stir the solution for a few seconds. Fill the test tube ¾ full with some liquid (no ice) from your beaker. Pour this cold liquid slowly into the cylinder and record your observations. 6. Clean the glassware. Analysis Questions-Salinity/Density: 1. What happened to the drop of green salt water solution in the tap water? Why? 2. What happened to the drop of blue tap water in the clear salt water solution? Why? 3. Why did the clear tap water and green salt water not mix together? 4. What happened when the two drops of red salt water solution were added to the different layers? Why? Analysis Questions-Temperature/Density: 1. Write a brief summary of your temperature/density experiment. 2. Given equal salinities, would cold or warm water have the greatest density? Explain your response. 3. Circle the correct answers in the following statements. Warm/Cool surface temperatures and high/low surface densities occur in the equatorial regions (regions close to the equator). At high latitudes, warm/cool surface temperatures and high/low surface densities are found.







