Lab Instructions - My Tummy Hurts
advertisement

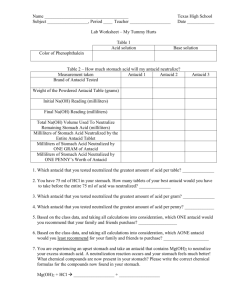
DO NOT WRITE ON THIS LAB!!!! LAB # ________ Lab Instructions – My Tummy Hurts Background: Your stomach is an amazing organ! The stomach begins the digestive process by secreting a very strong/concentrated Hydrochloric Acid with a pH of about 2. The inside of your stomach is normally protected from the strong HCl by a coating of mucous, but sometimes your stomach produces too much acid (like with you eat too much spicy food, or you are very stressed) that can eat through the mucous coating and actually start to digest your stomach lining. This hurts and burns causing “heartburn”, stomach aches, and in the worst cases a sore or ulcer forms. A quick relief for excess stomach acid is to tae an antacid. Antacids are really just bases (pH>7) that will react with and neutralize the acid (pH<7) in your stomach to produce salt and water (pH = 7). With the acid gone (or greatly reduced) the burning pain goes away and your stomach makes a new protective mucous lining! 1. You will be trained to titrate acids and bases using a buret. 2. You will be trained to calculate how much stomach acid an antacid will neutralize. 3. You will be trained to determine which antacid is the best buy. Materials: safety goggles 1 buret, ring stand, and buret clamp 1 funnel – must fit in buret 1 – 250 ml flask 1 -100 ml graduated cylinder 1 mortar and pestle with plastic spoon 1 – 250 ml beaker to hold Na(OH) balance weighing paper HCl Na(OH) Phenolphthalein with pipette Procedure: Part 1 – Phenolphthalein A. Phenolphthalein is an indicator that is commonly used in Chemistry. Like all indicator solutions, it can be used to give a continuous pH reading of a liquid and will let you know when a pH change has taken place by changing colors. B. Your teacher will demonstrate the color changes of Phenolphthalein. Record the color of Phenolphthalein in an acid solution and in a base solution in Table 1. Part 2 – The Upset Stomach A. You MUST keep your safety glasses on to protect you from the strong acids and bases used in this lab. B. Pick up a 250 ml Erlenmeyer flask. This will represent your stomach! Use a graduated cylinder to carefully measure out 50 ml of Hydrochloric Acid (HCl) and pour it in the flask. You have just eaten a large, spicy meal and your stomach is full of burning acid! C. Choose an antacid you would like to test and record its name in Table 2. D. Take a tablet of the chosen antacid and grind it into a powder using a mortar and pestle (this will help the antacid tablet dissolve in your stomach acid). E. Use a plastic spoon to scrape the powdered antacid tablet and weigh it (don’t forget to subtract the weight of the paper you should be using when you weigh a powder). Record the weight of the powdered tablet in Table 2. Clean and dry the mortar and pestle. F. Pour all the powdered antacid tablet into your “upset stomach” flask of acid. Gently swirl to help dissolve the antacid. G. You can’t really see what happened in your “upset stomach” as you added the antacid but a Neutralization Reaction quickly took place. Antacids are really just bases – like Mg(H)2. When the antacid’s base enters your stomach it reacts with the stomach’s acid (HCl) to produce harmless salt and water. There is now less acid in your stomach! GOOD ANTACIDS CONTAIN MORE BASE AND WILLNEUTRALIZE MORE OF YOUR STOMACH’S ACID! H. How did your antacid do? Remember, you started with 50 ml of HCl in your “stomach”. How much of this acid was neutralized by the antacid and how much acid remains in your “stomach”? Part 3 will help you answer these questions! Part 3 – Titrating With a Buret A. Add THREE drops of Phenolphthalein Indicator Solution to your stomach flask and observe. Are your stomach’s liquids still acidic (pH < 7) or did the antacid neutralize all the acid and turn your stomach’s liquids slightly basic (pH > 7)? B. The clear color in your “stomach” flask tells you the stomach liquid still has acid in it and the pH is less than 7. The antacid did NOT neutralize the entire 50 ml of HCl stomach acid. You still can’t tell if your antacid worked great (like the drawing below). Or not so great (like the drawing below). C. To find out exactly how well your antacid worked you are going to use a buret to add Na(OH) base to you “stomach” flask. A buret is like a large, expensive pipette on a stand that measures very accurately. The laboratory method you are going to use is called a titration. A titration is when you find the unknown concentration of one liquid called a Titration. A titration is when you find the unknown concentration of one liquid (the amount of HCl left in the “stomach”) by adding another liquid (Na(OH) base) to it until a color change occurs. D. Carefully move a buret to your table. Make sure the valve is in the OFF position so liquid will not come out the end (see Sketch A). You should also make sure there is a funnel in the top of your buret. E. Carefully bring a beaker of Na(OH) base to your table. Use the funnel to help you carefully fill the buret (from the top) with Na(OH) base, however, don’t try to fill it too fast or it will overflow. You do not need to fill the buret to the 0 ml line. Anywhere close to, but below, the 0 ml line is fine. NEVER FILL THE BURET WITH THE STOMACH ACID/ANTACID MIXTURE OR IT WILL CLOG THE BURET! F. Notice the lines on the side of the buret. The numbered lines are milliliters (ml) and the lines in between are tenths of a milliliter. These numbers tell you how many ml of liquid you have released from the buret – NOT how many ml of liquid are in the buret. Also notice that the top level of the Na(OH) in the buret tube dips down in the middle. When reading the volume of a liquid you should read from the bottom of this curve (see Sketch A). G. Place the beaker of Na(OH) beneath the tip of the buret. Make sure it is positioned to catch liquid as it escapes. Turn the valve to the ON position (see Sketch A) and let a few ml of Na(OH) escape from the tip of the buret into the Na(OH) beaker. This will remove any air from the buret tip. Notice that you can adjust the valve so that Na(OH) come out of the tip in slow drips if needed. H. Turn the valve to the OFF position and move the beaker of Na(OH) out of your way. Place your “stomach” flask beneath the tip of the buret. It’s time to titrate and find out how much HCl acid remains in your “stomach”! I. Look at the buret and determine the EXACT ml reading of Na(OH) in the tube (remember, you do not need to begin at 0 ml). This number is your Initial Na(OH) Reading. Record this reading in Table 2. J. Add a small amount (1 – 2 ml) of Na(OH) from the buret to your “stomach” flask, then gently swirl the flask. BE CAREFUL NOT TO BANG THE GLASS TIP OF THE BURET – IT BREAKS! The 1 – 2 ml of Na(OH) you just added will neutralize 1 – 2 ml of the HCl remaining in your “stomach” flask. Your goal is to neutralize ALL the remaining acid so you will know how much was there. Remember, the phenolphthalein you added to the “stomach” flask will turn pink the second all the acid is neutralized and the pH reaches 7. Continue to slowly add Na(OH) to the “stomach” flask until it turn pink and stays a light pink color for at least 15 seconds. All the acid is now neutralized!! K. Look at the buret and determine the EXACT ml reading of Na(OH) in the tube. This number is your Final Na(OH) Reading. Record this reading in Table 2. L. Calculate the Total Na(OH) Volume you used to neutralize the remaining “stomach” acid by using the following formula: Final Na(OH) Reading – Initial Na(OH) Reading = Total Na(OH) Volume used Example: Your Initial Na(OH) Reading was 3.2 ml and after neutralizing all the HCl your Final Na(OH) Reading was 28.6 ml. The Total Na(OH) Volume used was 28.6 ml – 3.2 ml = 25.4 ml M. Pour the neutralized “stomach” acid down the sink and rinse out the “stomach” flask. Part 4 – More Antacids! A. Repeat the titration process using a SECOND antacid of your choice. Ad more Na(OH) to the buret before beginning if needed, and don’t forget to add phenolphthalein to your “stomach” flask! Record all data in Table 2. B. Repeat the titration process using a THIRD antacid of your choice. Record all data in Table 2. C. Look at the data you recorded in Table 2. You should now have a rough idea about which of the antacids you tested neutralized the most stomach acid. After completing Part 5 you will know for sure which of the antacids is the best! D. When you have completed all your tests you should clean and return all your equipment. Your supervisor will tell you what to do with the buret. Part 5 – Calculating How Well Your Antacids Worked A. Look at the pictures below to understand how titrating with Na(OH) helped you determine the amount of stomach acid each antacid neutralized. B. You began each experiment with 50 ml of hydrochloric acid (HCl) in your “stomach”. How many ml of this acid did each antacid table neutralize? Calculate by using this formula: ml of stomach acid neutralized by the entire antacid table 50 ml of acid = to begin with = total Na(OH) volume used during titration to neutralize the remaining acid Record this calculation for each antacid in Table 2. C. It really isn’t fair to compare one antacid tablet to another because some tablet brands may work better simply because they are much larger than the others. To make fair comparisons you should calculate how much stomach acid can be neutralized by ONE GRAM of each antacid. Calculate by using this formula: ml of stomach acid neutralized = neutralized by 1 gram of antacid ml of stomach acid neutralized by the entire tablet weight (grams) of the powdered antacid tablet D. Do you pay for what you get? Do expensive antacid tables neutralize more acid than cheaper brands? To answer these questions you must calculate how much stomach acid can be neutralized by a single penny’s worth of each antacid. Calculate by using this formula: ml of stomach acid neutralized by 1 gram of antacid = ml of stomach acid neutralized by the entire tablet weight (grams) of the powdered antacid table Your teacher will supply you with the cost of antacid tablets. Record your calculations in Table 2.