Chemistry essay
advertisement

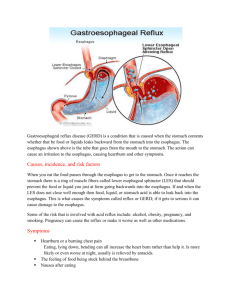
Chemistry Student #47 Mr. Ceccarelli Honors Chemistry I 4 January 2013 Antacids Heartburn is a common issue that many people face after eating a meal. It is that burning feeling attained after eating a meal that causes discomfort to many. For those who suffer, antacids are the first thing that comes to their mind. They know that a couple tablets or a swig of the stuff (depending on the form the antacid comes in) will alleviate the heartburn and make them feel better. What those people do not know is why heartburn is caused and how they are saved by simple chemistry. Heartburn actually has absolutely nothing to do with the heart. It is cause by stomach acid splashing against the bottom of the esophagus when the sphincter (the barrier between the esophagus and the stomach) is not closed properly and lets the acid through. The stomach naturally has a layer of mucus to protect itself from the acid; however, this is not as true for the esophagus which is why we feel a burning sensation. The hydrochloric acid (HCl) naturally created by the body eats away at the esophagus creating the burning pain. To stop this, the antacid is taken to neutralize the acid. When an acid is neutralized, it is reacted with a base to bring the pH level (measure of how acidy or basic a solution is on a scale of one to fourteen where seven is neutral like water) of your stomach closer to seven. The user takes the antacids to neutralize the bulk of the acid present and reduce the amount of acid in your stomach which lowers the overall pH of your stomach. The HCl has a molarity of 0.1 ( 0.1 mol per liter of solution) which means that although this strong acid will not dissolve your entire body, it can definitely cause some discomfort when it eats at your esophagus or stomach lining (if ulcers, or holes in the stomach’s protective mucus lining are present). How the antacid (base) neutralizes the HCl is fairly simple. Bases usually have an –OH-1 group attached (a common sign that tells that a chemical is basic although it is not always in the form of -OH-1) and acids have one or more –H+1 on them. The acids and bases must have these to react and neutralize. What happens in a neutralization reaction is that the –OH-1 and the –H+1 are pulled from the acid and base and join together to form water (H2O (l)) while the other elements involved in the reaction form other substances. As there are different brands, there are different bases each with a different strength and structure. If someone were to take Phillip’s® Milk of Magnesia, which has a chemical formula of Mg(OH)2, the resulting reaction would look like this: 2HCl(aq) + Mg(OH)2(aq) MgCl2(aq) + 2H2O(l) In the reaction, the water was formed and the remaining elements formed Magnesium chloride. Reactions for other products would be similar with a slightly different product. Because the reaction requires two mol HCl for every one mol Mg(OH) to be balanced, little of the antacid must be taken for the reaction to work (depending on the concentration). In all, antacids are taken mainly to neutralize the stomach acid that is eating away at sensitive tissue. When this happens, the acid and base react to form water and another substance, bringing the pH closer to seven in the process. After knowing what processes go on to relieve the burning sesation, people may stop to think not knowing the how and the why about heartburn and antacids. Works Cited "Antacids and Acid Reducers." WebMD. WebMD, 15 Dec. 2010. Web. 04 Jan. 2013. Brown, Theodore L., H. Eugene LeMay Jr., Bruce Edward. Bursten, and Catherine J. Murphy. Chemistry, the Central Science. 11e. Upper Saddle River, NJ: Prentice Hall, 2009. Print.