Agar Slant Innoculation
advertisement

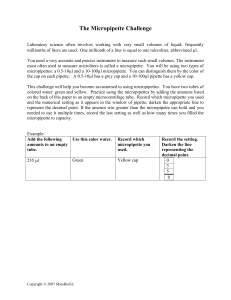
Procedure: Basic Lab Skills Micropipettes The unit of measurement is the microliter (µl). The capacity of each micropipette is indicated by a number usually on the top, expressed as a min/max or just a max. Note: Each micropipette is engineered to measure within a particular range of volumes. The range is usually given on the top of the pipet. For example 2 – 20 designates a functional range of 2 – 20 µl. Sometimes only the maximum range is given, i.e. P200 indicates that maximum range is 200 µl. In this case, the minimum range can often be inferred from the accompanying pipets in the set. If a P20 is being used, one would choose to NOTES: use the P20 instead of the P200 to measure accurately below 20 µl. One must be familiar with the pipets used in the particular lab to determine the appropriate pipet to use. CAUTION: DO NOT SET THE MICROPIPETTES FOR VOLUMES OUTSIDE THEIR RANGES. THIS WILL CAUSE MISCALIBRATION AND INACCURATE MEASUREMENTS. Setting the micropipette: Again, the technique for setting the micropipette varies with the manufacturer. Often there is a dial or in the housing of the micropipette and a widow with three digits. Turning the dial rotates the digits through cycles of ten to turn the next unit, much like an odometer. The digits represent different units by orders of ten depending on the range of the micropipette. For example, on a P20 the top digit represents the 10’s, middle digit 1’s and bottom digit 0.1’s. On the P200, the digit values are 100’s, 10’s, and 1’s respectively. Copyright © 2007 MassBioEd 1 Operation: 1. Always use a clean, disposable tip. 2. Depress the plunger to the first position. 3. Insert the tip in the liquid. 4. Slowly release the plunger. 5. Withdraw the pipette from the liquid. 6. Insert the pipette tip in the new tube. 7. Slowly depress the plunger to the second position. 8. With the plunger depressed, remove the pipette tip from the liquid. 9. Depress the eject button to remove the disposable tip when finished. Activity: Use the “Micropipette Challenge” to practice your pipette skills. Electronic Balances Operation: 1. Check that the balance is plugged in and turned on. 2. Position the balance away from drafts or vibrations. 3. Press the tare (zero) button. The display should read 0.00. 4. Place the object on the balance. 5. Read the mass in grams on the display. NOTES: If a container such as a weigh boat or beaker is used to contain a substance during measurement, be sure to tare the empty container before adding the substance. Activity: Measure 2 g of NaCl in a test tube. Save the NaCl for the next activity. Serological Pipettes The unit of measurement for serological pipets is milliliters. Most serological pipettes require a pump. Operation: 1. Insert the top of the pipette firmly into the base of the pump. 2. Check the pump plunger is in the fully depressed position. 3. Insert the tip of the pipet into a liquid. Copyright © 2007 MassBioEd NOTES: 2 4. Turn the wheel of the pump clockwise to draw the liquid up. 5. Dispense the liquid by turning the wheel counterclockwise. Activity: Add 10mL water to the container containing 2 g of NaCl. Vortex The vortex is used to mix solutions. Operation: 1. Set the toggle switch to touch. 2. Adjust the speed control to 5. 3. Hold the upper third part of the test tube firmly between your index finger and thumb. 4. Place the bottom of the test tube on the rubber platform and push the test tube down firmly. 5. Hold for 5 – 10 seconds. NOTES: Activity: Vortex the salt solution. Calculate the molarity of this solution? (Molecular weight of NaCl= 58.5 grams/mole) Molarity= Moles of solute / Liters of solution Glassware washing 1. 2. 3. 4. Pour the salt solution into a sink. Scrub the test tube with a test tube brush. Rinse the test tube 10 times with tap water. Rinse the test tube 3 times with distilled water. NOTES: Centrifuge The centrifuge is used to spin samples at high speeds resulting in a separation of components by mass. Activity: Obtain a microcentrifuge tube of E.coli. Centrifuge the E.coli solution to create a bacterial pellet. Copyright © 2007 MassBioEd 3 1. Check that the samples to be centrifuged are of equal size, mass, and volume. 2. Close the cover of the tubes. 3. Place the tubes in the rotor receptacles in a balanced configuration. Maintain an equal space between samples. Hint: Align the tube hinges facing out. The pellet should form on the same side as the hinges. 4. Close the cover of the centrifuge. 5. Adjust the timer to the desired time, usually in minutes. Note: Setting the timer to pulse will allow you to manually control the time of centrifugation. The rotor will only spin while the start button is depressed. 6. A red light will display when the rotor is turning. Note: If the centrifuge vibrates excessively, it is not balanced. Turn of the centrifuge immediately by turning the timer to 0 in timer mode or releasing the start button in pulse mode. 7. Wait for the red light to turn off, indicating that the rotor is stopped. 8. Open the cover and remove the samples. Indicate the proper balancing of a centrifuge when you have 3 microcentrifuge tubes Label the supernatant and pellet in the diagram. Sterile Technique We avoid using flames with students in the lab. Therefore our sterile technique uses disposable sterile tips and transfer loops. A general rule of thumb for sterile technique is to breach sterile boundaries as briefly as possible. Resuspension: 1. Obtain a container labeled “Bacterial Waste.” 2. Hold the tube containing the E.coli pellet at a 45 degree angle. (A 45 degree angle minimizes exposure to particulates in the air.) 3. Open the tube and pour the supernatant into the container labeled “Bacterial Waste.” 4. Close the tube. Copyright © 2007 MassBioEd 4 Resuspension Con’t.: 5. Use a sterile pipette tip to add 200 µl of sterile water to the E.coli pellet. Remember to hold the tubes at 45 degrees. 6. Resuspend the E.coli pellet by gently drawing the pellet in and out of the pipette tip until the solution is cloudy with no visible E.coli chunks. 7. Cap the tube and dispose of the pipette tip in the bacterial waste container. NOTES: Pouring Agar: 1. Lift the lid of a Petri dish just enough to admit the neck of the bottle. Avoid touching the bottle to any surface. 2. Pour the enough agar to cover the bottom of each dish. 3. Cover the dish and let it sit until the agar is hardened. (about 30 – 45 min.) Note: Cover should not be off Inoculating liquid cultures 1. Obtain an agar dish with viable bacteria. 2. Obtain 1 ml of sterile Luria Broth (with ampicillin for this exercise). 3. Obtain a packaged sterile transfer loop. 4. Open the end of the package that does not contain the loop. 5. Remove the transfer loop, being careful to touch only the handle. Copyright © 2007 MassBioEd 5 6. Open the cover of the agar dish with bacteria just enough to allow a sterile loop to touch the surface of the agar. 7. Gently drag the transfer loop across the surface of the agar, collecting bacterial colonies on the loop. You may see clump of bacteria on the end of the loop. However, it is not necessary to see the bacteria. 8. Remove the loop and insert it into the 1 ml of Luria Broth. Remember to hold the tube at an angle. 9. Spin the loop in the broth to release bacteria into the solution. 10. Remove the transfer loop, close the tube, and dispose of the loop in the bacterial waste. Sterile Technique Activity Check your sterile technique by transferring 100 µl of sterile water to a sterile agar dish. Procedure: 1. Obtain a microcentrifuge tube of sterile water. 2. Obtain a sterile Petri dish of LB agar. 3. Label the bottom of the dish “sterile water” along with your initials and today’s date. 4. Use a micropipette with a sterile tip to transfer 100 µl of sterile water to the agar. 5. Spread the water with a sterile transfer loop by gently moving the sterile loop across the agar. Agar Slant Innoculation a. Unwrap a sterile applicator swab. b. Use it to collect some E. coli bacteria from the E. coli agar plate. c. Inoculate agar slants by gently swiping the swab on the agar. Recap the agar slants and incubate at room temperature for 4 days, or in a 37ºC incubator for 2 days, until bacterial colonies are visible. Once colonies are present, the slants can be stored in a 4oC refrigerator. Copyright © 2007 MassBioEd 6 The Micropipette Challenge Using 0.5-10µl and 10-100µl Micropipettes Laboratory science often involves working with very small volumes of liquid; frequently millionths of liters are used. One millionth of a liter is equal to one microliter, abbreviated l. You need a very accurate and precise instrument to measure such small volumes. The instrument most often used to measure microliters is called a micropipette. You will be using two types of micropipettes: a 0.5-10µl and a 10-100µl micropipette.. You can distinguish them by the color of the cap on each pipette. A 0.5-10µl has a grey cap and a 10-100µl pipette has a yellow cap. This challenge will help you become accustomed to using micropipettes. You have two tubes of colored water: green and yellow. Practice using the micropipettes by adding the amounts listed on the back of this paper to an empty microcentrifuge tube. Record which micropipette you used and the numerical setting as it appears in the window of pipette; darken the appropriate line to represent the decimal point. If the amount was greater than the micropipette can hold and you needed to use it multiple times, record the last setting as well as how many times you filled the micropipette to capacity. Example: Add the following amounts to an empty tube. Use this color water. Record which micropipette you used. 235 l Green Yellow cap Copyright © 2007 MassBioEd Record the setting. Darken the line representing the decimal point. 0 3 5 0 7 Amount to add in empty tube Color of Water 220 l Green 7 l Green 74.4 l Yellow .0183 ml Green .583 ml Yellow 197 l Yellow .0047 ml Green 48.6 l Yellow 347 l Green Copyright © 2007 MassBioEd Record micropipette used Record the setting by darkening the line representing the decimal point 8 How many l should you have in test tube when you are finished? How many ml should you have in the test tube when you are finished? Can you think of a way to use the 10-100µl micropipette to check that the amount of liquid in your test tube is what it should be? Did you pipet the correct amount of liquid? If not, what happened? Copyright © 2007 MassBioEd 9 Micropipette Challenge - Answer Key and Notes Each group of students needs a 0.5-10µl and 10-100µl micropipe as well as the correct tips for each micropipette. If p1000's are not available, you can modify the activity by having students skip the volumes above 200 l, which are in the 1st, 5th, and 9th rows. Answer Correct pipetting will result in a final volume of 1500 l or 1.5 ml. Students can check their pipetting accuracy in two ways: 1. Most microcentrifuge tubes have marks at 0.5 ml, 1ml, and 1.5 ml. Their liquid should be at the level of the 1.5 ml mark, and the tube should be almost full. 2. Students can measure the liquid in their tube with a 1ml pipette. Withdraw 1 ml of liquid from the tube. Expel it into a liquid waste container. Then withdraw the rest of the liquid in the tube into the pipet. It should read 0.5ml. The Micropipette Challenge Using the p1000, p200 and p20 Series Laboratory science often involves working with very small volumes of liquid; frequently millionths of liters are used. One millionth of a liter is equal to one microliter, abbreviated l. You need a very accurate and precise instrument to measure such small volumes. The instrument most often used to measure microliters is called a micropipette. You will be using three types of micropipettes: a p1000, a p200, and a p20. You can distinguish them by the label on each pipette. A p1000 is used to measure volumes from 201l to 1000l (equal to 1ml), a p200 is used to measure volumes from 21l to 200l, and a p20 is used to measure volumes from 2l to 20l. This challenge will help you become accustomed to using micropipettes. You have two tubes of colored water: green and yellow. Practice using the micropipettes by adding the amounts listed on the back of this paper to an empty microcentrifuge tube. Record which micropipette you used and the numerical setting as it appears in the window of pipette; darken the appropriate line to represent the decimal point. Example: Add the following amounts to an empty tube. Use this color water. Record which micropipette you used. 35 l Green p200 Copyright © 2007 MassBioEd Record the setting. Darken the line representing the decimal point. 0 3 5 0 10 Copyright © 2007 MassBioEd 11 Amount to add in empty tube Color of Water 220 l Green 7 l Green 74.4 l Yellow .0183 ml Green .583 ml Yellow 197 l Yellow .0047 ml Green 48.6 l Yellow 347 l Green Record micropipette used Record the setting by darkening the line representing the decimal point How many l should you have in test tube when you are finished? Copyright © 2007 MassBioEd 12 How many ml should you have in the test tube when you are finished? Can you think of a way to use the p1000 to check that the amount of liquid in your test tube is what it should be? Did you pipet the correct amount of liquid? If not, what happened? Copyright © 2007 MassBioEd 13 Micropipette Challenge - Answer Key and Notes Each group of students needs a p20, a p200, a p1000, and the correct tips for each micropipette. If p1000's are not available, you can modify the activity by having students skip the volumes above 200 l, which are in the 1st, 5th, and 9th rows. Answer - Using a p20, a p200, and a p1000 Correct pipetting will result in a final volume of 1500 l or 1.5 ml. Students can check their pipetting accuracy in two ways: 3. Most microcentrifuge tubes have marks at 0.5 ml, 1ml, and 1.5 ml. Their liquid should be at the level of the 1.5 ml mark, and the tube should be almost full. 4. Students can measure the liquid in their tube with the p1000. Set the p1000 to1000l and withdraw 1000 l from the tube. Expel it into a liquid waste container. Now set the p1000 to 500 l and withdraw 500 l from the tube. If they have air bubbles or empty space in the tip, they did not pipet the full 1.5 ml. If they have extra liquid in the tube after removing 1000 l and 500 l, they pipetted more than 1.5 ml. Answer - Using a p20 and a p200 Correct pipetting of the six volumes less than 200 l will result in a final volume of 350 l. Students can check their pipetting accuracy in two ways: 1. Most microcentrifuge tubes have marks at 0.5 ml, 1ml, and 1.5 ml. Their liquid should be a bit below the level of the 0.5ml mark. 2. Students can check their final volume using the p200. Set the p200 to 200 l and withdraw 200 l from the tube. Expel it into a liquid waste container. Now set the p200 to 150 l and withdraw 150 l from the tube. If they have air bubbles or empty space in the tip, they did not pipet the full 350 l. If they have extra liquid in the tube after removing 200 l and 150 l, they pipetted more than 350 l. Copyright © 2007 MassBioEd 14