Tube 1
advertisement
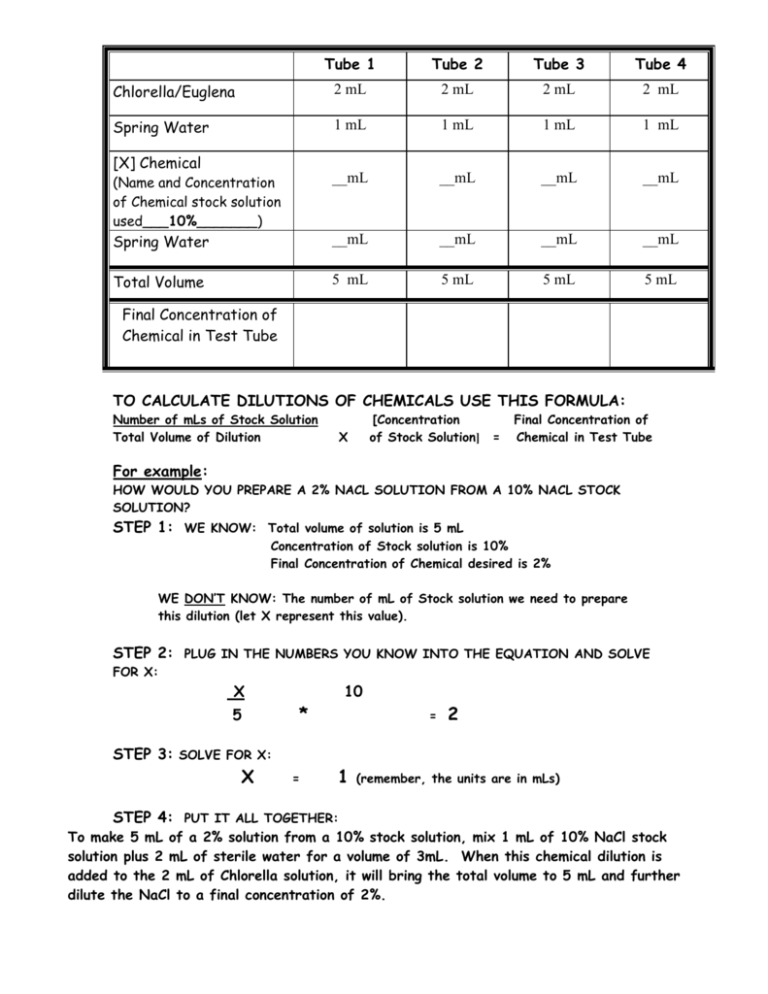
Tube 1 Tube 2 Tube 3 Tube 4 Chlorella/Euglena 2 mL 2 mL 2 mL 2 mL Spring Water 1 mL 1 mL 1 mL 1 mL (Name and Concentration of Chemical stock solution used___10%_______) __mL __mL __mL __mL Spring Water __mL __mL __mL __mL Total Volume 5 mL 5 mL 5 mL 5 mL [X] Chemical Final Concentration of Chemical in Test Tube TO CALCULATE DILUTIONS OF CHEMICALS USE THIS FORMULA: Number of mLs of Stock Solution Total Volume of Dilution [Concentration of Stock Solution] X = Final Concentration of Chemical in Test Tube For example: HOW WOULD YOU PREPARE A 2% NACL SOLUTION FROM A 10% NACL STOCK SOLUTION? STEP 1: WE KNOW: Total volume of solution is 5 mL Concentration of Stock solution is 10% Final Concentration of Chemical desired is 2% WE DON’T KNOW: The number of mL of Stock solution we need to prepare this dilution (let X represent this value). STEP 2: PLUG IN THE NUMBERS YOU KNOW INTO THE EQUATION AND SOLVE FOR X: X 5 10 * = 2 STEP 3: SOLVE FOR X: X = 1 (remember, the units are in mLs) STEP 4: PUT IT ALL TOGETHER: To make 5 mL of a 2% solution from a 10% stock solution, mix 1 mL of 10% NaCl stock solution plus 2 mL of sterile water for a volume of 3mL. When this chemical dilution is added to the 2 mL of Chlorella solution, it will bring the total volume to 5 mL and further dilute the NaCl to a final concentration of 2%.