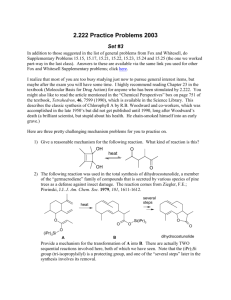
Evaluation of Tailor-Made Polymeric Supports
advertisement

Polymer Journal Supplementary Information for Synthesis and evaluation of resins bearing substrate-like inhibitor functions for capturing copper amine oxidases Marco Pocci,* Silvana Alfei, Sara Castellaro, Francesco Lucchesini, Marco Milanese and Vincenzo Bertini Dipartimento di Farmacia, Università di Genova Via Brigata Salerno, 13 I-16147 Genova, Italy. *Corresponding author: Marco Pocci Tel.: +39 010 3532685; Fax: +39 010 3532684 E-mail: pocci@dictfa.unige.it Contents 1. Synthesis of 2,6-dimethoxy-4-hydroxybenzaldehyde (4) 2. Synthesis of 2,6-dimethoxy-4-hydroxybenzaldoxime (7) 3. Synthesis of 2,6-diethoxy-4-hydroxybenzaldoxime (8) 4. Synthesis of 2,6-dimethoxy-4-hydroxybenzonitrile (9) 5. Synthesis of 2,6-diethoxy-4-hydroxybenzonitrile (10) 6. Synthesis of (E)-2,6-dimethoxy-4-[(2-tetrahydropyranyloxy)-2-butenyloxy]benzonitrile (12) 7. Synthesis of (E)-2,6-diethoxy-4-[(2-tetrahydropyranyloxy)-2-butenyloxy]benzonitrile (13) 8. References 1. Synthesis of 2,6-dimethoxy-4-hydroxybenzaldehyde (4) OEt HO OEt DMF/POCl3 rt HO OEt -10 °C OEt OEt CH=O OEt 4 (50%) + O=HC + HO OEt CH=O 5 (26%) HO OEt CH=O 6 (11%) A mixture of 3,5-diethoxyphenol [1] (3.52 g, 19.3 mmol) and POCl3 (5.67 g, 37.0 mmol, 3.4 mL) was cooled to -10 °C, treated dropwise with DMF (2.14 g, 29.3 mmol, 2.3 mL), stirred under N2 at rt for 24 h 40 min then poured in iced water and treated with solid NaOH (7.01 g, 175.3 mmol) up to pH = 9. The solution was acidified with 12% aq. HCl 1 (pH = 6-7) to give a solid precipitate which was filtered, dried over P 2O5, washed with CHCl3 (2x10 mL) and crystallized from MeOH to afford 2,6-diethoxy-4-hydroxybenzaldehyde (4) (2.02 g, 50%). Purity 99% by HPLC. Mp: 178-180 °C. FTIR (KBr, cm-1): 3218 (OH); 1659 (CH=O). 1H-NMR (DMSO-d6, δ ppm): 1.33 (t, 6H, J = 7.0 Hz); 4.00 (q, 4H, J = 7.0 Hz); 6.06 (s, 1H); 10.20 (s, 1H); 10.50 (s, broad OH). 13C-NMR: 14.33, 63.82, 92.44, 107.04, 162.84, 164.77, 185.31. The CHCl3 washings after removal of the solvent left 2,4-diethoxy-6-hydroxybenzaldehyde (5) (1.06 g, 26%). For characterization purposes a sample was sublimed at reduced pressure in the form of a white waxy solid. Purity 98% by HPLC. Mp: 70-71°C. FTIR (KBr, cm-1): 1643 (CH=O). 1H-NMR (CDCl3, δ ppm): 1.41 (t, 3H, J = 7.0 Hz); 1.43 (t, 3H, J = 7.0 Hz); 4.048 (q, 2 H, J = 7.0 Hz); 4.052 (q, 2 H, J = 7.0 Hz); 5.88 (d, 1H, J = 2.0 Hz); 5.97 (d, 1H, J = 2.0 Hz); 10.10 (d, 1H, J = 0.4 Hz); 12.50 (s, 1H). 13C-NMR: 14.42, 14.48, 64.04, 64.17, 91.44, 93.17, 105.94, 162.91, 166.26, 167.47, 191.87. 2- Hydroxy-4,6-diethoxy-1,3-benzenedicarboxaldehyde (6) was obtained by slow spontaneous precipitation from the aqueous phase of the hydrolytic step (0.536 g, 11%). Purity 99% by HPLC. Mp: 168-171 °C. FTIR (KBr, cm-1): 3427 (OH); 1690 (CH=O). 1H-NMR (CDCl3, δ ppm): 1.51 (t, 6H, J = 7.0 Hz); 4.20 (q, 4H, J = 7.0 Hz); 5.91 (s, 1H); 10.22 (s, 1H); 13.47 (s, OH). 13C-NMR: 14.35, 65.15, 86.94, 106.07, 167.76, 168.32, 189.44. 2. Synthesis of 2,6-dimethoxy-4-hydroxybenzaldoxime (7) OMe CH=O HO OMe 3 OMe CH=NOH NH 2OH . HCl abs EtOH reflux Pyridine HO OMe 7 (90%) A mixture of 3 [2,3] (2.98 g, 16.4 mmol), EtOH 100% (35 mL) and pyridine (3.5 mL) was treated under stirring at rt with NH2OH·HCl (1.37 g, 19.7 mmol) to afford an orange suspension which was refluxed for 3 h and left at rt overnight. After removal of the solvent at reduced pressure the solid residue was crystallized from EtOH 95% to give 7 as an orange solid (2.91 g, 90%). Purity 97% by HPLC. Mp: 210-218 °C (dec.). FTIR (KBr, cm-1): 3316, 3234 (OH), 1597 (C=N). 1H-NMR (DMSO-d6, δ ppm): 3.72 (s, 6H); 6.12 (s, 2H); 8.13 (s,1H); 9.93 (s, broad, OH); 10.67 (s, very broad, OH). 13C-NMR: 55.45, 92.03, 100.74, 142.56, 159.24, 159.98. 3. Synthesis of 2,6-diethoxy-4-hydroxybenzaldoxime (8) OEt CH=O HO OEt 4 OEt NH 2OH . HCl abs EtOH reflux Pyridine CH=NOH HO OEt 8 (78%) A solution of 4 (2.44 g, 11.6 mmol), EtOH 100% (28.5 mL) and pyridine (2.9 mL) was treated as described for 7 with NH2OH·HCl (0.967 g, 13.9 mmol). After 2 h reflux the solvent was removed at reduced pressure to afford 8 which was crystallized from EtOH 90% (2.02 g, 78%). Purity 97% by HPLC. Mp: 150-152 °C. FTIR (KBr, cm-1): 3411 (OH), 1611 (C=N). 1H-NMR (DMSO-d6, δ ppm): 1.30 (t, 6H, J = 7.0 Hz); 3.96 (q, 4H, J = 7.0 Hz); 6.07 (s, 2H); 8.12 (s, 1H); 9.76 (s, broad, OH), 10.59 (s, broad, OH). 13C-NMR: 14.56, 63.61, 92.98, 101.42, 142.91, 158.55, 159.71. 2 4. Synthesis of 2,6-dimethoxy-4-hydroxybenzonitrile (9) OMe CH=NOH HO OMe 7 OMe C N Cu(OAc)2 CH 3CN reflux HO OMe 9 (77%) A mixture of 7 (3.52 g, 17.9 mmol), CH3CN (19 mL) and a solution of Cu(OAc)2 (0.376 g, 2.1 mmol) in CH3CN (19 mL) was heated under N2 with stirring for 2 h 45 min until disappearance of 7 (HPLC). The brown suspension was hydrolyzed with 5% H2SO4 (100 mL), extracted first with Et 2O (3x30 mL) then with EtOAc (3x30 mL). The extracts were combined, washed with H2O then dried (Na2SO4).The removal of the solvent at reduced pressure afforded 9 (2.45 g, 77%). Purity 97% by HPLC. Mp: 261-262 °C (CH3CN); FTIR (KBr, cm-1): 3222 (OH), 2228 (CN); 1H-NMR (DMSO-d6, δ ppm): 3.81 (s, 6H); 6.14 (s, 2H); 10.69 (s, OH). 13C-NMR (δ, ppm): 55.97, 80.70, 92.08, 114.67, 163.32, 164.02. 5. Synthesis of 2,6-diethoxy-4-hydroxybenzonitrile (10) OEt HO OEt CH=NOH Cu(OAc)2 OEt CH 3CN reflux 8 C N HO OEt 10 (70%) A mixture of 8 (1.03 g, 4.6 mmol), CH3CN (4.5 mL) and a solution of Cu(OAc)2 (0.092 g, 0.51 mmol) in CH3CN (44 mL) was heated under N2 with stirring for 1 h 30 min until disappearance of 8 (TLC, petroleum ether/EtOAc = 1/2 as eluent). The blue solution was hydrolyzed with 5% H2SO4 (30 mL), extracted first with Et 2O (30 mL) then with EtOAc (2x30 mL). The extracts were combined, washed with H 2O then dried (Na2SO4). The removal of the solvent at reduced pressure afforded crude 10 which was crystallized from toluene as a yellow solid (0.658 g, 70%). Purity 99% by HPLC. Mp: 173-175 °C. FTIR (KBr, cm-1): 3247 (OH), 2222 (CN). 1H-NMR (DMSO-d6, δ ppm): 1.34 (t, 6H, J = 7.0 Hz); 3.34 (s, OH); 4.06 (q, 4H, J = 7.0 Hz); 6.11 (s, 2H). 13C-NMR: 14.25, 64.15, 81.26, 92.62, 114.64, 162.55, 163.83. 6. Synthesis of (E)-2,6-dimethoxy-4-[(2-tetrahydropyranyloxy)-2-butenyloxy]benzonitrile (12) OMe C N HO OMe 9 OMe C N 1) NaH/DMF 2) THPO THP O Cl O OMe 12 (63%) A solution of benzonitrile 9 (3.70 g, 20.7 mmol) in dry DMF (38.5 mL) was added under N2 to a suspension of NaH [0.933 g (23.3 mmol) as 60% mineral oil dispersion, washed with pentane (3x2 mL)] in dry DMF (8 mL). The suspension was left under stirring at rt for 1 h 30 min, cooled to 0 °C, treated with (E)-1-chloro-4-(2tetrahydropyranyloxy)-2-butene (11) [4] (5.79 g, 30.4 mmol, 5.5 mL), refluxed for 3 h, hydrolyzed with H 2O (150 mL) and extracted with Et2O (3x50 mL). The extracts were combined, washed with 10% NaOH and dried (Na2SO4). The removal of the solvent at reduced pressure left a residue (7.69 g) which was subjected to column chromatography using 3 a mixture EtOAc/petroleum ether = 40/60 as eluent to give 12 as an oil (4.38 g, 63%). Purity 99% by HPLC. FTIR (film, cm-1): 2219 (CN), 970 (CH=CH, trans). 1H-NMR (CDCl3, δ ppm): 1.52-1.95 (m, 6H); 3.47-3.58 (m, 1H); 3.833.90 (m, 1H); 3.85 (s, 6H); 4.00-4.07 (s, 1H); 4.27-4.34 (m, 1H); 4.57-4.60 (m, 2H); 4.63-4.68 (m, 1H); 5.94-6.03 (m, 2H); 6.09 (s, 2H). 13C-NMR: 19.45, 25.41, 30.57, 56.12, 62.30, 66.66, 68.45, 84.26, 91.09, 98.20, 114.55, 125.98, 131.29, 163.77, 164.29. The last fractions of the column eluted with 100% EtOAc allowed the isolation of solid (E)-2,6-dimethoxy-4-[4hydroxy-(2-butenyloxy)]benzonitrile (0.46 g, 9%). Purity 98% by HPLC. Mp: 201-202 °C. FTIR (KBr, cm-1): 3412 (OH), 2216 (CN). 1H-NMR (Acetone-d6, δ ppm): 2.89 (s, OH); 3.91 (s, 6H); 4.10- 4.17 (m, 2H); 4.66-4.72 (m, 2H); 5.85-6.10 (m, 2H); 6.33 (s, 2H). 13C-NMR: 56.71, 62.33, 69.45, 84.44, 92.32, 114.64, 124.55, 135.85, 164.59, 165.53. 7. Synthesis of (E)-2,6-diethoxy-4-[(2-tetrahydropyranyloxy)-2-butenyloxy]benzonitrile (13) OEt HO 10 C N 1) NaH/DMF OEt 2) THPO OEt C N Cl THP O O OEt 13 (82%) A solution of benzonitrile 10 (2.00 g, 9.7 mmol) in dry DMF (13 mL) was added under N2 to a suspension of NaH [0.44 g (11.0 mmol) as 60 % mineral oil dispersion, washed with pentane (3x2 mL)] in dry DMF (3.7 mL). The suspension was left under stirring at rt for 1 h 30 min, cooled to 0 °C, treated with 11 [4] (2.71 g, 14.2 mmol, 2.6 mL), heated at 70 °C for 4 h 30 min, hydrolyzed with H 2O (75 mL) and extracted with Et2O (3x25 mL). The extracts were combined, washed with 10% NaOH and dried (Na2SO4). The removal of the solvent at reduced pressure left a residue (4.19 g) which was subjected to column chromatography using a mixture EtOAc/petroleum ether = 40/60 as eluent to give 13 as oil (2.89 g, 82%). Purity 99% by HPLC. FTIR (film, cm-1): 2220 (CN), 970 (CH=CH, trans). 1H-NMR (Acetone-d6, δ ppm): 1.40 (t, 6H, J = 7.0 Hz); 1.45-1.85 (m, 6H); 3.42-3.48 (m, 1H); 3.74-3.83 (m, 1H); 3.94-4.02 (m, 1H); 4.15 (q, 4H, J = 7.0 Hz); 4.17-4.27 (m, 1H); 4.61-4.64 (m, 1H); 4.65-4.69 (m, 2H); 5.88-6.07 (m, 2H); 6.27 (s, 2H). 13C-NMR: 14.74, 19.95, 26.17, 31.21, 62.15, 65.36, 66.98, 69.09, 84.87, 92.73, 98.41, 114.62, 126.77, 131.86, 163.72, 165.13. The last fractions of the column eluted with 100% EtOAc allowed the isolation of solid (E)-2,6-diethoxy-4-[4-hydroxy(2-butenyloxy)]benzonitrile (0.0944 g, 4%). Purity 99% by HPLC. Mp: 98-100 °C. FTIR (KBr, cm-1): 3476 (OH), 2230 (CN). 1H-NMR (Acetone-d6, δ ppm): 1.40 (t, 6H, J = 7.0 Hz); 3.85-4.12 (m, 2H); 4.17 (q, 4H, J = 7.0 Hz); 4.65-4.68 (m, 2H); 5.88-6.06 (m, 2H); 6.30 (s, 2H). 13C-NMR: 14.79, 62.33, 65.48, 69.39, 85.09, 92.96, 114.65, 124.59, 135.81, 163.88, 165.40. 8. References 1. Maillard J, Vincent M, Delaunay P, Fumeron B (1996) Derivés du phloroglucinol. 1. Synthèse de mono, di et triéthers d’acides trihydroxy-2,4,6-benzoiques mono et dichlorés, et leur acétylation. Bull. Soc. Chim. Fr. (8): 25202524 2. Albericio F, Kneib-Cordonier N, Biancalana S, Gera L, Masada RI, Hudson D, Barany G (1990) Preparation and application of the 5-(4-(9-fluorenylmethyloxycarbonyl)aminomethyl-3,5-dimethoxyphenoxy)valeric acid (PAL) handle for the solid-phase synthesis of C-terminal peptide amides under mild conditions. J. Org. Chem. 55(12): 3730-3743 4 3. Jin J, Graybill TL, Wang MA, Davis LD, Moore ML (2001) Convenient preparation of 4-formyl-3,5dimethoxyphenol and its incorporation into linkers and resins for solid-phase synthesis. J. Comb. Chem. 3(1): 97-101 4. Deslongschamp P, Lamothe S, Ho-Shen-Lin (1987) A simple and direct method of cyclization for the synthesis of 10-membered rings. Can. J. Chem. 65(6): 1298-1307 5