Marina Ulanova, MD, PhD - Ontario Lung Association
advertisement

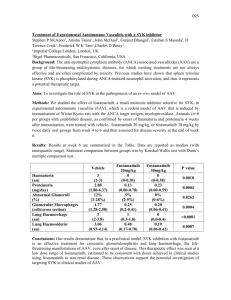
Marina Ulanova, MD, PhD Dr. Marina Ulanova is a full time tenured faculty member in the Medical Sciences Division, Northern Ontario School of Medicine (NOSM) at the Lakehead University campus (Thunder Bay, Ontario). In addition to teaching Fundamentals of Medicine for medical students, Dr. Ulanova is involved in the supervision of undergraduate and graduate Lakehead University students enrolled in various programs (Biology, Chemistry, Health Sciences, Applied Bio-Molecular Science, PhD Program in Biotechnology). She also holds a faculty appointment at the Laurentian University (Faculty of Medicine and the Biomolecular Sciences PhD program). Dr. Ulanova obtained her MD from the Pirogov Memorial Moscow State Institute of Medicine and her MSc and PhD in Immunology/Pediatrics from the Institute of Pediatrics, Russian Academy of Medical Sciences (Moscow, Russia). She obtained a second PhD in Clinical Immunology from the University of Gothenburg (Sweden). After relocating to Canada in 2000, she completed postdoctoral training in Dr. Dean Befus’ group at the University of Alberta. She has been an Associate Professor at NOSM since 2005. Since her early studies in pediatric immunology in Russia, Dr. Ulanova has always been fascinated with the remarkable immunological mechanisms which protect the respiratory system against any potential insults. She studied immunological mechanisms of respiratory diseases for many years and at different levels, ranging from the role of secretory immunoglobulins in mucosal immune defense against acute respiratory infections in the 1980s, and specific immune response to pneumococcal serotypespecific antigens causing pneumonia in children in the 1990s, to signal transduction mechanisms mediating asthma attacks during her postdoctoral training in the 2000s. Her current research concerns the molecular mechanisms underlying the interactions between pathogenic bacteria and lung epithelial cells with specific emphasis on innate immune and inflammatory responses induced by the opportunistic pathogen Pseudomonas aeruginosa. She is also interested in vaccine immunology, particularly in the effect of pediatric vaccination against Haemophilus influenzae type b (Hib) and Streptococcus pneumoniae on modern immunoepidemiology of invasive H. influenzae and S. pneumoniae disease, i.e. changing host-pathogen interactions and microbial ecology in the post-vaccine era. In collaboration with clinical faculty at NOSM, she investigates immune response of immunodeficient patients to encapsulated bacterial pathogens. In addition, she studies epidemiology of community acquired pneumonia with particular emphasis on predisposing immunological factors in the Aboriginal population of Northern Ontario. The Ontario Thoracic Society had supported Dr. Ulanova’s research through the OTS Grants-In-Add Program. When she takes a break from her busy schedule, Dr. Ulanova greatly enjoys the unique natural environment of Northern Canada. She spends her free time kayaking, hiking, cross-country skiing, and doing nature photography. The Role of Syk In Acute Pulmonary Infection Caused by Pseudomonas Aeruginosa Principal Investigator: Marina Ulanova, MD, PhD Introduction The opportunistic bacteria Pseudomonas aeruginosa cause severe lung damage in patients with ventilator-associated pneumonia (VAP) in intensive care units.1 The overall mortality rate for P. aeruginosa associated VAP is 69%, with septic shock being the most common immediate cause of death.2 Although it is evident that an excessive inflammation of infected cells in the lung is the reason for the detrimental tissue damage caused by P. aeruginosa infection, the underlying molecular mechanisms are poorly defined. Since the non-receptor protein tyrosine kinase Syk was discovered in lung epithelial cells several years ago,3 researchers have been puzzled by the role of this molecule in the lung. It is well established that Syk is a critical component of immunoreceptor signaling in hematopoietic cells and its activation in leukocytes is essential for phagocytosis and the development of B- and T-lymphocytes. Syk also regulates innate immune responses including pathogen recognition, inflammasome activation, and anti-fungal defense.4 Our recent studies as well as the research conducted by other scholars demonstrated the ability of Syk to regulate production of pro-inflammatory molecules by bronchial epithelial cells stimulated with a cytokine TNF-α 5,6 or infected by human rhinovirus.7 We hypothesized that Syk is critically involved in the regulation of inflammatory responses caused by P. aeruginosa infection and that its inhibition may alleviate the process of lung tissue destruction during this infection. To test this hypothesis we used a selective Syk inhibitor piceatannol, which is a naturally occurring non-toxic bioactive compound present in various plants. Methods We infected Syk-positive H292 or Syk-negative A549 lung epithelial cell lines with P. aeruginosa and assessed the resulting cellular responses, i.e. production of proinflammatory cytokines, adhesion molecule expression, generation of reactive oxygen species (ROS), and apoptosis of infected cells, using multiplex bead-based immunoassay and flow cytometry. We also studied internalization of P. aeruginosa using the gentamicin exclusion assay. To assess the involvement of Syk, we pretreated cell cultures with a low dose of piceatannol considered to be Syk-selective. Results & Discussion P. aeruginosa infection caused potent activation of inflammatory responses of infected cells, i.e. release of pro-inflammatory cytokines IL-2, IL-6, IL-8, IFN-γ, GM-CSF, TNF-α, along with the up-regulation of the expression of ICAM-1, an adhesion molecule involved in the recruitment of activated leukocytes into inflamed lung tissue. Moreover, the infected cells produced large amounts of intracellular ROS and underwent apoptosis, reproducing an in vivo infectious process when P. aeruginosa induce the oxidative stress of infected cells enhancing inflammation and tissue damage. As ROS can initiate apoptosis of epithelial cells infected with P. aeruginosa,8 such mechanism may further contribute to lung injury. We found that piceatannol treatment significantly suppressed inflammation, oxidative stress, and apoptosis in H292, but not in A549 cells, implicating Syk participation in the regulation of the pathological processes induced by P. aeruginosa infection. Considering that normal lung epithelium, i.e. bronchial epithelial cells, as well as type I and II pneumocytes express large amounts of Syk¸3 its activation during P. aeruginosa infection may be significantly involved in the pathogenesis of acute lung injury caused by this pathogen. Intriguingly, piceatannol was able to down-regulate the internalization of P. aeruginosa by both Syk-positive, and Syk-negative cell lines implying that the mechanisms of action of this compound, even when used at a low concentration, may extend beyond Syk inhibition. Clinical Relevance Our findings provide a rationale for the use of specific inhibitors of Syk tyrosine kinase to alleviate two central components in the pathogenesis of acute P. aeruginosa pulmonary infection, i.e. inflammation and tissue damage. The effect of piceatannol on P. aeruginosa infection is apparently more complex than expected, and may involve targeting multiple signaling pathways, apart from Syk-dependent ones. As piceatannol can interfere with several mechanisms of bacterial pathogenesis, including Sykindependent internalization, this natural compound deserves further study as a potential therapeutic option in P. aeruginosa infection. References 1. Garau J and Gomez L: Pseudomonas aeruginosa pneumonia. Curr Opin Infect Dis (2003) 16:135-43. 2. Crouch Brewer S, Wunderink RG, Jones CB, et al: Ventilator-associated pneumonia due to Pseudomonas aeruginosa. Chest 1996, 109:1019-29. 3. Ulanova M, Duta F, Puttagunta L, et al: Spleen tyrosine kinase (Syk) as a novel target for allergic asthma and rhinitis. Expert Opin Ther Targets 2005, 9:901-21. 4. Mócsai A, Ruland J, Tybulewicz VLJ: The SYK tyrosine kinase: a crucial player in diverse biological functions. Nature Rev Immunol 2010, 10:387-402. 5. Ulanova M, Puttagunta L, Marcet-Palacios, et al: Syk tyrosine kinase participates in β1-integrin signaling and inflammatory responses in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 2005, 288:L497-507. 6. Ulanova M, Marcet-Palacios M, Munoz S, et al: Involvement of Syk kinase in TNFinduced nitric oxide production by airway epithelial cells. Biochem Biophys Res Commun 2006, 351: 431-7. 7. Wang X, Lau C, Wiehler S, et al: Syk is downstream of intercellular adhesion molecule-1 and mediates human rhinovirus activation of p38 MAPK in airway epithelial cells. J Immunol 2006, 177:6859-70. 8. Jendrossek V, Grassme H, Mueller I, Lang F, Gulbins E: Pseudomonas aeruginosainduced apoptosis involves mitochondria and stress-activated protein kinases. Infect Immun 2001, 69: 2675-83. Research Review 2011; Volume 8