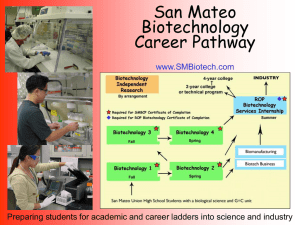
Biotech 1 Course Outline
advertisement

Biotechnology I Course Outline 2015-2016 A. Biotechnology Industry (15%) BTI 01.00 Understand Organizational Structure of the Biotech Industry (14%) 1.01 Remember Historical Events and People A. 1600s Leeuwenhoek B. 1860 Mendel C. 1919 Karl Ereky D. 1943 Penicillin produced on industrial scale E. 1953 Watson and Crick F. 1961 – 1966 Genetic Code deciphered G. 1973 Boyer and Cohen H. 1978 Genentech produced human insulin in E. coli I. 1980 Diamond v Chakrabarty Supreme court case J. 1981 First monoclonal diagnostic kit approved for US K. 1984 DNA fingerprinting technique developed L. 1988 Polymerase chain reaction (PCR) method published M. 1997 First cloned mammal N. 2003 Human genome project completed 1.02 Understand Types of Biotech Companies A. Review via web sites and visits to biotechnology companies the organizational structure of various biotechnology and pharmaceutical companies 1. Organizational structure: CEO, COO, CFO, CIO, VP of research, etc 2. Types of companies: research, pharmaceutical, manufacturing, multi-disciplined 3. Identify the different types of products and applications provided by biotech companies: medical, agricultural, environmental, industrial, marine, other 1.03 Understand Organizational Structure and Career Options Research career options and job descriptions for various jobs in the biotechnology industry including educational requirements and skills required A. Regulatory Affairs B. Research Scientist C. Manufacturing technician D. Bio-processing engineer E. Quality Assurance F. Quality Control G. Statistician H. Clinical Affairs I. Lawyer: patent, general counsel, compliance officer J. Metrology K. Materials department and inventory control Explore biotechnology career opportunities A. Manufacturing B. Quality Control / Quality Assurance C. Regulatory D. Sales and Marketing E. Research F. Forensic Pathology G. Forensic Psychiatry H. Other areas Create a personal career development plan A. Career goal B. Educational requirements C. Work experience / Volunteer opportunities D. Financial plan 1. Cost of tuition 2. Scholarship opportunities 3. Personal budget plan Update College or Job Applications A. Complete a job application B. Complete a college application C. Complete a financial aid or loan application B. Basic Laboratory Skills (60%) BTI02.00 Understand Safety Requirements of Working in a Biotech Lab (10%) 2.01 Understand Laboratory and Personal Care Safety A. Hand washing technique B. Gowning technique C. Personal Protection Equipment D. Lab Surface cleaning procedure E. Fire safety F. Proper handling of spills G. Glassware cleaning procedure H. Waste disposal procedure I. Aseptic Technique (lab and manufacturing) 2.02 Introduce students to laboratory notebooks A. Notebook format B. Documentation requirements 1. Pen 2. Date and Initial 3. Title of experiment or lab C. Grading requirements 2.03 Understand Roles of OSHA, EPA and DOT as related to Biotech Analyze Material Safety Data Sheets (MSDS) A. Proper handling and storage of chemicals through lab set up B. Establish and review all MSDS sheets and their importance C. Learn how to request an MSDS from a supplier D. Practice reading an MSDS for various types of materials Outline Federal Agencies that Regulate Safety and Environmental Protection A. Occupational Health and Safety Administration (OSHA) B. Environmental Protection Agency (EPA) C. Department of Transportation (DOT) BTI03.00Understand Basic Science Behind Biotechnology (10%) 3.01 Understand cell structure of Prokaryotes and Eukaryotes Review differences between eukaryotes and prokaryotes A. Prokaryotes B. Eukaryotes Review of cellular structure and organelle function A. Mitochondria B. Cytoplasm C. Ribosome D. Cell Membrane E. Nucleus F. Golgi apparatus G. Vacuoles H. Chloroplasts Explain differences between viruses and Bacteriophages A. Viruses 1. Consist of genetic material enclosed in a capsule generally made of protein. 2. Require a host cell to make copies of themselves 3. When a virus infects a cell it introduces genetic material into that cell 4. Extremely specific about the host cell they can infect 5. Limited to certain cell types as well as host types B. Bacteriophages 1. Bacterial virus specific to certain bacteria strains 3.02 Understand the role of biological molecules Discuss the Four Classes of biological molecules A. Proteins and Enzymes B. Carbohydrates C. Nucleic Acids D. Lipids 3.03 Demonstrate the techniques necessary to grow cells in culture A. Media preparation B. Sterile technique C. Triple Z inoculation BTI04.00 Apply Procedures for Making Buffers (25%) 4.01 Understand the basic concepts of metrology A. Units of measurement B. Accuracy vs precision C. percent error and standard deviation D. standards, verification, calibration, traceability E. tolerance, errors, and uncertainty 4.02 Apply proper use of pipettes, balances, vortexers, and pH meters for buffer manufacture Learn the art of Pipetting A. Explain the various pipets and pipetting techniques for lab experiments 1. Fixed Volume pipets 2. Disposable pipets 3. Adjustable volume pipets 4. Micropipets 5. Multi channel pipets B. Explain how and why pipets are calibrated 1. Maintain calibration records of pipets C. Explain the preventive maintenance for pipets 1. Maintain preventive maintenance records D. Explain the use of various balances for weighing 1. Analytical balances 2. Floor balances 3. Pan or top load balances E. Practice using various pieces of equipment 1. Explain significant figures F. Explain how and why balances are calibrated 1. Maintain routine calibration sheets in the lab 2. Verification before use 3. Use of traceable standards G. Explain the preventive maintenance for balances Demonstrate Buffer Preparation B. Explain Solution Preparation 1. Practice making mass/volume solutions 2. Practice making percent solutions 3. Practice making molar solutions 4. Practice making dilutions from stock solutions 2. Demonstrate serial dilutions C. Explain pH and its importance 1. Acids 2. Bases 3. Neutral 4. Buffers 5. Titrations D. Practice using various methods of measuring pH 1. pH meters 2. pH paper E. Explain how and why pH meters are calibrated 1. Maintain routine calibration sheets in the lab F. Explain the preventive maintenance for pH meters BT105.00 Apply Skills for Using Laboratory Equipment (15%) 5.01 Apply skills for proper use of Spectrophotometers and chromatography techniques Understand how to use a Spectrophotometer A. Explain the use of the spectrophotometer 1. Visible 2. Ultra Violet B. Practice using various pieces of equipment 1. Protein standard curve assays 2. Colorimetric assays 3. Absorption spectrum C. Explain how and why spectrophotometers are calibrated 1. Maintain routine calibration sheets in the lab D. Explain the preventive maintenance for spectrophotometer Explore physical properties and molecular polarity A. The causes of molecular polarity B. Attractions between molecules C. Solubility properties of substances D. The melting process E. The boiling process Demonstrate extractive separation and chromatography A. Paper and Thin Layer Chromatography B. Column chromatography options Demonstrate the basic use of gel electrophoresis A. Agarose gel electrophoresis to separate DNA or dyes B. Importance of buffers and dyes 5.02 Apply skills for proper use of Microscopes and Centrifuges Demonstrate proper use of the Microscopes A. Explain the use of the microscope 1. Objectives 2. Stage 3. Condenser 4. Ocular (eye piece) 5. Course adjustment 6. Fine adjustment B. Practice using various pieces of equipment 1. Prepared Gram stains 2. Pond water 3. Prepared blood smears C. Explain the preventive maintenance for microscope Explore Centrifugation A. Explain the use of various centrifuges 1. Micro-centrifuge 2. Bench top centrifuge 3. 2-8oC centrifuge B. Practice using various pieces of equipment 1. Explain balancing a centrifuge 2. Explain rpm and its importance C. Explain how and why centrifuges are calibrated 1. Maintain routine calibration sheets in the lab D. Explain the preventive maintenance for centrifuges C. Regulation of the Biotechnology Industry (15%) BTI06.00 Understand FDA Role in Biotech Industry (8%) 6.01 Understand the organizational structure and roles of the FDA Explore the Role of the FDA A. Food Drug and Cosmetic Act B. Food and Drug Administration 1. Identify various products regulated by the FDA C. Good Manufacturing Practices 1. Review various warning letters 2. Review 483s from inspections Explore the Organization of the FDA A. Center for Drug Evaluation and Research (CDER) B. Center for Biologics Evaluation and Research (CBER) C. Center of Devices and Radiological Health (CDHR) D. Center for Food Safety and Applied Nutrition (CFSAN) E. Center for Veterinary Medicine F. Center for Tobacco Products 6.02 Understand the basics of Good Laboratory Practices Good Laboratory Practices A. Why they were developed B. Who must follow them Explain and demonstrate Good Lab Practices (GLP) Understand and write Standard Operating Procedures A. How to read, write and follow an SOP B. Understanding what a batch record is C. Completing a batch record according to GMP D. Storage and handling of documents 6.03 Understand clinical trials and Good Clinical Practices Design a clinical trial A. Review the various phases of a clinical trial B. Incorporate the use of blinded samples C. Define roles 1. Primary investigator 2. Data reviewers D. Define planned statistical analysis E. Select clinical trial site and participants F. Define required approvals: Internal Review Board Execute and monitor clinical trial A. Gather data B. Monitor participants Summarize Data Analysis A. Analyze data using statistical processes B. Comment on outliers, mean, standard deviation BTI07.00 Understand Quality Systems Regulations in the Biotech Industry (7%) 7.01 Understand current Good Manufacturing Practices and basics of standard operating procedures Explain Good Manufacturing Practices and Quality System Regulations A. Define Good Manufacturing Practices B. Define Quality System Regulations 1. Review of the QSR standards 2. Differences between 9000, 9001, 9002 certifications 3. Pros and Cons of certifications Explain the labeling requirements for product A. Product Labels B. Package inserts Explain the approval requirements for marketing literature A. Marketing materials Explain Audit Elements A. Training B. Document Control C. Material Storage and Handling C. Bioethics (10%) BTI08.00 Understand bioethical issues involved in biotechnology (10%) 8.01 Understand the bioethical viewpoints A. Stem cell research B. Genetically modified organisms C. Biofuels D. Privacy issues on DNA manipulation E. Organ transplantation F. Assisted reproductive technology G. Assisted end of life H. Uses of animals in research I. Biological patents