Alumina Sample Clean-up
advertisement

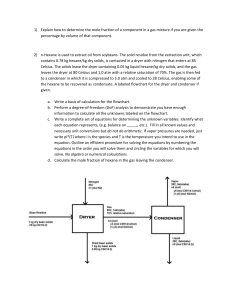
Fractionation of Samples for PCB, PAH, and OC Pesticide Analysis Using Solid-Liquid Chromatography with Alumina as the Stationary Phase Prerequisite: The knowledge of possible ways to contaminate the samples and the hazards of working with these organic pollutants, as well as the solvents involved in the procedure must be known. Scope and Application: This procedure is performed to fractionate the samples into polychlorinated biphenyl, polycyclic aromatic hydrocarbon, and organochlorine pesticide (OC-pesticide) for further analysis. Summary: The samples that were extracted in dichloromethane (DCM), petroleum ether, or acetone-hexane (1:1 mix), and reduced in volume to 1 mL, are run through a chromatographic column of alumina and sodium sulfate, pre-eluted with both a DMC-hexane mix and hexane alone. The PCBs are collected in the first fraction (F1), along with some of the OC-pesticides and the PAHs and the remainder of the OC-pesticides are collected in the second fraction (F2). The two fractions are then volume reduced and prepared for either GC/MS or GC/ECD analysis. Apparatus: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. Serological pipettes (baked overnight) 50 mL centrifuge tubes (baked overnight) Sodium Sulfate (baked at 450C for 4 hours) Glass Wool (cleaned through solvent extraction) Alumina (deactivated, see SOP for “Alumina Preparation”) Autoclave Tape and markers Stand with clamps to hold the pipettes Pasteur Pipettes for solvent and pipette tops (2mL) ~50 mL beakers Erlenmeyer flask (for DCM:hexane mixture) 50 mL graduated cylinders Aluminum weighing trays 1. 2. 3. Dichloromethane Hexane Petroleum Ether Reagents: Safety: Safety glasses and nitrile gloves must be worn at all times during this procedure. If sample or solvent spills on your body, immediately rinse with soap and water. Procedure: 1. Preparation a. Combine a 2:1 mixture of Dichloromethane/Hexane in an Erlenmeyer flask. (Quantity based on number of samples) b. Rinse two centrifuge tubes with hexane and label them with the sample code and F1 or F2 for each sample to be cleaned up. c. Place and pack a small amount of glass wool into a 10ml x 1/10 serological pipette. NOTE: be careful to pack the glass wool tightly enough to not allow any alumina to fall through the bottom, but not so tightly that the solvent drips through at an astonishingly slow pace. d. Weigh out 4.00 grams of deactivated alumina on an aluminum-weighing pan. NOTE: Rinse weighing pans with petroleum ether before weighing e. Add weighed alumina to the wool packed pipette. f. Pack the alumina in the column by gently tapping the column with a pen. g. h. i. Add a pinch of sodium sulfate to the top of the packed column. Place a 50mL beaker under the column to collect the pre-elution solvents. Measure 5 ML of 2:1 DCM:hexane mix into the graduated cylinder (NOTE: it is imperative that the column NEVER go dry, always add liquid to the column when the previous elution has reached the top of the sodium sulfate layer). j. Precondition the column with this 5ml using the hexane-rinsed Pasteur pipettes with pipette tops. k. Measure 15 mL of hexane into the graduated cylinder. l. When the mix reaches the top of the sodium sulfate… m. Recondition the column with this 15 ml of hexane. n. When the 15 mL of hexane is in the column, refill the graduated cylinder with 13 mL of hexane. 2. Chromatography a. When the hexane reaches the top of the sodium sulfate… b. Using a hexane-rinsed Pasteur pipette place sample on the head of the column. c. Place the centrifuge tube labeled F1 under the column. d. Rinse vial with ~1ml hexane drawing in from the 13 ml of hexane. Use a smooth motion, rinsing the sides of the vial that contained the sample. e. Add the hexane rinse once the sample drains into the sodium sulfate layer of the column. f. Repeat this rinse procedure two more times (for a total of three rinses). Only add the rinse after the previous one has reached the top of the sodium sulfate. g. Allow final rinse to reach the sodium sulfate, and then add the rest of the 13 mL of hexane to the column. h. Fill the graduated cylinder with 15 mL 2:1 DCM/hexane mixture. i. When the hexane from the first fraction has reached the sodium sulfate layer, switch the F1 labeled tube with the F2 labeled centrifuge tube. Place the F1 tube in a rack and cover it with foil and a cap. j. Start adding all the 15 ml of DCM/Hexane mixture. k. If doing a third fraction, add 15 ml of 2:1 Ethyl Acetate/Methanol Mixture when the DCM/Hexane mixture has reached the top of the sodium sulfate and exchange the F2 centrifuge tube for one labeled “F3.” l. Once all the solvent has eluted through the column, remove the F2 centrifuge tube, place foil over the top and add a cap. Keep the tubes in the sample freezer until you are ready to prepare them for a GC/MS or ECD run. Quality Assurance and Quality Control: Lab blanks and Matrix spikes are “cleaned-up” using this same procedure. The comparison between these and the samples’ surrogate recoveries helps determine if compounds were lost during any part of the extraction procedure.