LAB 7: THIN LAYER AND GAS CHROMATOGRAPHY
advertisement

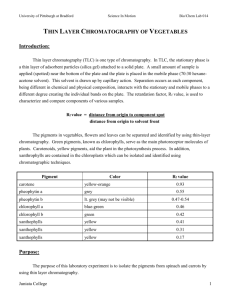
LAB 7: THIN LAYER AND GAS CHROMATOGRAPHY PRE-LAB: 1. Complete the Safety section for Lab 7. 2. What are the factors that affect how much of a solid will dissolve in a particular solvent? 3. Define the term chromatography. 4. What are the properties of a good eluent for carrying out TLC? PURPOSE: You will learn how to separate complex mixtures of small samples of solids using thin layer chromatography and to separate complex mixtures of solids, liquids, and/or gases using gas chromatography. Thin layer chromatography experiments of an analgesic drug mixture will be performed and gas chromatography will be demonstrated. BACKGROUND INFORMATION: Historically, the term chromatography referred to the separation of colored substances, particularly plant pigments. Over time, many variations of chromatography were developed and the technique extended to colored and colorless substances in any physical state (solid, liquid or gas). In general, the technique is based on the different absorption characteristics of substances on some non reactive material. A series of absorptions and de-absorptions occur as some eluting material is passed through the chromatography container. The many types of chromatography include: 1. Thin Layer (TLC) - best for very small samples of solids or liquids. 2. Gas (GC) - best for small samples of volatile liquids or gases. 3. Column - best for larger samples of solids or liquids (see next experiment). 4. High Pressure Liquid (HPLC) - best for larger samples of liquids. 5. Paper - best for biological or polar samples. 6. Ion Exchange - best for ionic substances. 7. Electrophoresis - best for large molecule biological samples. THIN LAYER CHROMATOGRAPHY - TLC started to be used in many laboratories in the late 1950's or early 1960's. This technique (which is generally qualitative and not preparative) uses a stationary phase of silica gel or alumina which is adhered to a backing material of plastic, glass or (less frequently) metal. Often an ultraviolet absorbing substance is placed in the binder material on the plate so that spots can be more easily visualized when the chromatogram is finished. The TLC plate is usually 2 by 4 inches to 8 by 8 inches. A concentrated solution (using methanol as the solvent) of the mixture to be separated is prepared and spotted on the TLC plate about 3/4 of an inch from the bottom using a spotting capillary pipette made from a melting point tube. The spot is made as small as possible. After the methanol solvent has evaporated, the TLC plate in placed in a developing chamber which has an eluting solvent that is appropriate to separate the components of the mixture. Capillary action causes the eluent to rise up the TLC plate. The plate is removed from the developing chamber when the eluent is about 1/2 inch from the top of the plate. The eluting solvent is allowed to evaporate in a fume hood and the chromatogram is studied in an ultraviolet light chamber to see the separation that has been obtained. The location of a given spot is recorded as an Rf value (retardation factor) which is given by the formula shown above. An Rf value has no units and always has a value between 0 and 1.0. In today's experiment, you will try to separate a mixture of analgesics whose structures appear below using TLC. b Rf = a distance spot moved a = b distance solvent moved Today we will be performing TLC on some known analgesics. Note the similarities and differences in the molecules. Known Analgesics: CH3 CH CH3 CH2 O C OH H O O CH3 H3C C COOH Ibuprofen 2-(4-Isobutylphenyl)propionic acid Aspirin Acetylsalicylic acid H O N C HO O CH3 H3C O CH3 N N N N CH3 Acetaminophen 4-acetamidophenol Caffeine GAS CHROMATOGRAPHY - GC is a technique that can be used to separate the components of a volatile (liquid or gas) mixture. Usually the method is qualitative in that components can be identified but not collected for further use. To perform a GC one uses an instrument made for the purpose. Such instruments can cost from $1,000 to $50,000 dollars depending on the abilities and complexity of the instrument. In order to use this instrument it must be set up correctly. The temperature of three areas needs to be set and come to equilibrium. These areas are the injection port, the column oven and the detector area. The injection port is usually set at a temperature above the highest boiling component. The oven temperature (which can be programmed in more expensive equipment) is set to give the best separation in the quickest time. Usually this takes some experimentation to determine the best values. Generally, the higher the oven temperature, the faster the substance will be removed from the column and the worse the separation of components. The detector temperature is set at a value to reduce condensation in that chamber. The separation of the components of the mixture depends on many factors that can be controlled by the operator of the instrument. The stationary phase in the column must be chosen so that a good separation is possible. Generally, columns are chosen and purchased for a particular type of separation. The oven temperature is very important as noted above. The pressure of the carrier gas (usually helium or nitrogen) is also important. Higher gas pressures move the substances along more quickly. One must achieve a balance between speed of the chromatogram and the separation that is desired. The detector identifies when a substance has been eluted from the column. In some cases, the detector can give further information about the nature of the eluted material. The simplest (and least expensive) detector is a thermal conductivity detector and can only identify that a substance has passed the detector. More sensitive detectors include flame ionization and electron capture detectors. The best detectors are the mass spectrometry detectors which can give detailed information about the eluted substance. Usually in GC one obtains a strip chart recording of the chromatography results on a piece of paper. A series of peaks of varying heights is obtained. Peaks appear at certain time intervals called the retention time. The retention time is determined by many experimental factors and is not recorded in the literature as a physical property of a substance. Rather, they are used to infer some information about the nature of the mixture. If you suspect the identity of a component of the mixture, you should run a chromatogram of a known sample and compare the results. The retention times should be the same if the substances are the same. Also, keep in mind that two different compounds may have the same retention time and will appear as one peak on the chromatogram. This can give misleading results. EXPERIMENTAL PROCEDURE: First the instructor or lab personnel may demonstrate the use of a gas chromatograph. 1) Obtain 2 TLC strips (keep the strips away from moisture), 5 spotting capillaries and 1 developing chamber. 2) Place 2 to 6 ml of the developing solution (47.5% ethyl acetate, 2.5% acetic acid and 50% hexane) in the developing chamber and place a cover on the chamber. Be sure there is enough developing solution in the bottom of the chamber to wet the entire bottom of TLC strip and for capillary action to wet the entire strip during development. Do not allow the chamber to be open to the atmosphere any longer than absolutely necessary as volatility will alter the composition of the solvent mixture. You may wish to use a fresh sample of the developing solution for your second run. Be careful not to mix spotting point capillaries and place them in the discarded glass container when you are finished. 3) Since the silica gel strips are reasonably expensive, each team will receive a practice strip and 2 TLC strips for the experiment. Practice your spotting technique on the practice strip. 4) Carefully spot pure samples and the unknown on the silica gel TLC strips, one sample at a time. Be sure to place each spot high enough so that it will be above the developing solvent in the bottom of the chamber when the strip is placed in the chamber. Be sure each spot is as small as possible (about 1 mm in diameter) and accurately record what you are placing on each spot. On each strip, place three known samples and your unknown sample. Use acetaminophen, aspirin, and caffeine as your choice for known samples. 5) Develop the chromatogram and remove the strip when the solvent front stops moving up the strip or when the solvent front is about ½ inch from the top of the strip. 6) Evaporate the eluting solvent and visualize the spots using a UV lamp. Carefully draw a light pencil circle around each spot so they can be seen in normal room light. (If your plate is difficult to read, check with the instructor for advice and repeat the procedure.) Draw a sketch of the plate in your lab notebook. 7) Calculate the Rf values for each spot and record this in your notebook. Compare the Rf values of your unknown sample to those of your known standards in order to identify the unknown, if possible. Check the identity with the instructor. 8) Dispose of the capillary tubes in the glass waste. The used TLC plates should be placed in the solid waste bin in the hood. Excess solvents should go in the organic waste container. IMPORTANT INFORMATION ABOUT THE REPORT: 1. Below are 3 TLC plates that were likely not run correctly. What might have been done wrong, if anything? A B C 2. How can you verify the identity of a compound using TLC. 3. For the reaction: A + B C a) Before the reaction has occurred to any extent, imagine that a small amount of the reaction mixture was spotted on a TLC plate and visualized. How many spots do you expect to observe? b) Imagine that A travels farther up the TLC plate than B. Sketch what this imaginary TLC plate might look like. c) Imagine that the reaction has progressed and the reactants are partially converted into C. If a TLC plate were spotted with the reaction mixture now, how many spots do you expect to observe? d) Can you determine which spot corresponds to each component? How? e) When the reaction is completed, what do you expect a TLC of the reaction mixture to look like? Sketch it. 4. Write a results section and a discussion section. Write your report about the thin layer chromatography experiment only; do not include anything in your report about gas chromatography. In your report, give the Rf values of each substance and discuss the conclusions you are able to make about the unknown. Identify your unknown and discuss how your arrived that that conclusion. Don’t forget diagrams/figures. END OF EXPERIMENT. © 2011 Carberry and Carreon