An Examination of Penicillin Allergy
advertisement

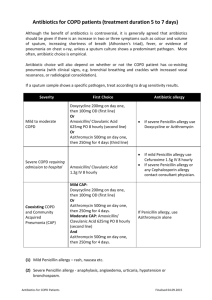
An Examination of Penicillin Allergy I. Introduction As many as 10% of the general population claims to be allergic to penicillin. In reality only 1-3% of people have an IgE-mediated reaction to penicillin. In recent years, the number of available antibiotics has increased dramatically. This has led physicians to avoid penicillins if a patient reports any adverse reaction; no matter how mild. Consequently, there has been increased use of fluoroquinolones and vancomycin, in particular. The concomitant rise in antimicrobial resistance and health care costs has prompted the medical community to reexamine the issue of penicillin allergy. A thorough review of this topic should answer the following questions: II. Are all patients that claim to be allergic to penicillin truly allergic? How can we determine who is truly allergic? Are there some infections for which penicillin is superior to other antibiotics? How would the increased use of penicillin help with antimicrobial resistance and health care costs? How does this apply to other β-lactams such as cephalosporins? Are there certain populations of patients that would benefit from routine skin testing? Is there a practical approach to patients with penicillin allergy? What is a true penicillin allergy? Like many pharmacologic agents, penicillin is simple in structure and of low molecular weight. Low molecular weight substances that are able to produce an allergic response are known as haptens. By themselves they are unable to induce antibody formation. In order to induce an immune response they must be attached to carrier molecules that form a strong covalent bond. Patients do not exhibit an immune response to penicillin itself but rather to the breakdown products of penicillin (isomers) after they bind with tissue and plasma proteins to form haptencarrier complexes. Many, if not all, of the breakdown products of penicillin are created in the vial, tablet, or powder prior to administration to the patient. The b -lactam ring in the degraded penicillin is unstable and binds with lysine residues in the tissue and plasma proteins resulting in a penicilloyl epitope known as benzylpenicilloyl (BSO) or the "major determinant". This major determinant is produced in the largest amount and therefore is immunodominant in penicillin specific immune responses. Although less common, the b -lactam rings can also make molecular rearrangements with carboxyl and thiol groups and form less dominant or "minor determinants" (Figure 1). The terms major and minor refer only to the amount of hapten available for binding and not to the importance of each hapten in an immunologic response. These complexes of penicillin break down products and native proteins are recognized as foreign antigens by mast cells and basophils in some individuals. Subsequently, when a sufficient density of drug epitopes is formed, a drug-specific immune response ensues. Minor determinants appear to cause 90-95% of immediate IgE-mediated reactions. Major determinants can cause an immediate IgE-mediated reaction but more often cause accelerated or delayed reactions caused by IgG and IgM. This is a very important clinical distinction because patients can have IgE-mediated reactions to the minor determinant alone and if skin tests only include the major determinant those patients (who are more likely to have an anaphylactic reaction) will not be identified. Note that prior to 1970, penicillin preparations were often contaminated with trace quantities of macromolecules and drug polymers (ampicillin), which increased the frequency of allergic reactions. The more recent "purified" penicillin preparations reduced but did not eliminate these reactions. A variety of infections create an inflammatory response that can enhance drug allergy. Rashes are common in patients with HIV, hepatitis B, coxsackie virus, echovirus and numerous bacterial infections. Therefore, patients with infections who take penicillin and develop a rash should not automatically be labeled with penicillin allergy. Figure 1. Immunochemistry of penicillin molecules. (from Adkinson et al.) Immediate Reactions (Type I) The penicillin-protein antigen complex (often the minor determinant) binds to fixed IgE on mast cells and basophils, triggering the release of mediators of inflammation such as histamine, serotonin, and prostaglandins. This results in increased capillary permeability (edema), changes in smooth muscle tone, and increased production of viscid mucous in target tissues. This usually occurs within one hour and clinically is manifested by any combination of diffuse erythema, pruritus, urticaria, angioedema, bronchospasm, laryngeal edema, hyperperistalsis, hypotension, and cardiac arrhythmias. These reactions occur in 0.004% - 0.015% of penicillin courses - most often in adults between the ages of 20 and 49 years. A history of atopic reactions increases the risk for an anaphylactic reaction. Immediate reactions are more common in parenteral administration. Fatal outcomes occur in 1 per every 50,000 to 100,000 treatment courses. Of note, it is possible for IgE-mediated reactions to occur from 1 to 72 hours after administration of penicillin. These are called "accelerated reactions" and are more often associated with the major determinant. They can result in urticaria, angioedema, laryngeal edema, and/or wheezing. Life threatening reactions occurring beyond 1 hour of penicillin administration are rare. Late Reactions (Type II, Type III, Type IV, idiopathic) These reactions occur after 72 hours of drug administration and are classified as Type II, III, or IV based on the underlying immune response. Because none of these reactions is IgE dependent, skin testing has no role in the evaluation of these patients. (Refer to Table I for a full summary). Table 1: Classification of Penicillin Reactions Classification Time of Onset Type I (immediate) < 1 hour Late reactions Variable Mediators Clinical Signs Skin Testing Useful? Commen IgE mediated crosslinkage with mast cells and basophils with release of vasoactivemediators. Anaphylaxis and/or hypotension, laryngeal edema, wheezing, angioedema, urticaria Yes More likely if parenteral ad Fatal in 1 per 100k treatme Type II IgG, complement Blood transfusion rxn., autoimmune hemolytic anemia No IgE not involv Type III IgG, IgM immune complexes Serum sickness, Tissue injury (glomerulonephritis, RA, SLE) No Tissue lodgin immune complexes; d fever Type IV Cytokines activate Tc cells causing direct cellular damage Contact dermatitis, TB lesions, Graft rejection. No Maculopapular or morbilliform rash No Idiopathic III. Usually > 72 h How can we determine who is truly allergic? The role and utility of skin testing in various clinical settings. Two basic classes of skin tests are used in clinical allergy practice – epicutaneous and intracutaneous (intradermal). The scratch and the prick tests are epicutaneous. The epicutaneous tests are more specific but less sensitive than the intradermal tests. The scratch test is performed by scratching the superficial epidermis with a needle or sharp instrument and applying a drop of antigen to the local abrasion. The prick test is performed by placing a drop of antigen 1%-4% of all patients rece penicillin on the skin and inserting a needle at a 45˚ angle through the drop to lift the epidermis. Intradermal testing is performed with a 26 to 30 gauge needle, creating a skin wheal of 0.02 cc of extract. Because the degree of cutaneous reactivity varies between patients, a diluent negative control and a positive control of histamine are used with the three methods. The reactions are read in 15 minutes and graded on a scale of negative to 4 +. The wheal is measured at its largest diameter and at the perpendicular diameter, and an average of the two is calculated. One plus is erythema up to 2 cm in diameter, 2+ is greater than 2 cm in diameter, 3+ reactions are erythema and wheal formation, and 4+ is erythema, wheal formation, and pseudopod formation. Reactions of 2+ or greater are considered significant. It is generally accepted that skin testing with both major and minor determinants is necessary in order to have the most sensitive and specific test possible because a minority of patients will have an adverse reaction to the minor determinant alone (often anaphylaxis). Currently, there is no commercial source for the minor penicillin skin test reagent in the US. Some use fresh penicillin G and its subsequent break down products as a minor determinant mixture. In recent months there have also been problems with the manufacture of major determinants. Centers that wish to skin test must custom make their materials or receive permission from the FDA to import them from Germany. Skin tests take approximately 40 minutes and the reagents and equipment cost approximately $17 per patient. The use of skin testing and an examination of its safety, cost, risks, and how predictive it is of penicillin allergy has been examined in a variety of settings. The following is a review of several of these studies. 1. Outpatient STD clinic Gadde J. et al. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA. (1993) Purpose: To determine the prevalence of positive penicillin skin tests among outpatients with well-defined but variable history of penicillin allergy and to determine the reproducibility, safety, and negative predictive value of skin testing with major and minor determinant mixtures. Design: Serial consenting outpatients with current indications for penicillin therapy were skintested with major and minor determinants. Those with negative skin tests received therapeutic benzylpenicillin or ampicillin. Negative predictive value of skin testing was established by 72 hour follow-up for adverse reactions to drug. Methods: 5063 consecutive patients in a Baltimore, MD STD clinic. 66% male, 90% AfricanAmerican. Follow-up was 94% complete. Results: Positive skin tests were found in 7.1% of 776 individuals with a previous history of penicillin allergy and in 1.7% of 4287 individuals negative by history. A previous history of anaphylaxis or urticaria was associated with a significantly higher rate of positive skin tests (17.3% and 12.4% respectively). The interval from last use of penicillin did not influence skin test results. Thirteen patients had an adverse reaction to the skin test itself (one had "mild anaphylaxis", 11 had urticaria, one had a large local reaction). After penicillin administration among patients with a negative skin test, acute allergic reactions occurred in only 0.5% (18/3997) of subjects negative by history. Three patients had an immediate reaction and 15 patients had an accelerated reaction. No patients had anaphylaxis. 2.9% (17/596) of patients with a negative skin test and a positive history had an acute allergic reaction. Reactions were typically mild with only 2 patients having "mild anaphylaxis" – both of these patients had a history of IgE-mediated reactions and responded to epinephrine. No deaths occurred. Of the patients with a positive skin test, 90% demonstrated major-determinant sensitivity but 10% reacted to minor determinants alone. Ninety-seven percent of those with a history of "penicillin allergy" were able to tolerate full dose penicillin therapy after a negative skin test. Conclusions: Skin testing with both major and minor determinants is safe and both reagents are needed to maximize the identification of sensitized subjects since minor determinants are associated with anaphylaxis. Penicillin skin testing can facilitate the safe use of penicillin in 90% of those patients with a previous history of penicillin allergy. 2. Hospitalized adults (non-ICU settings): Sogn D.D. et al. Results of the National Institute of Allergy and Infectious Diseases collaborative clinical trial to test the predictive value of skin testing with major and minor penicillin derivatives in hospitalized adults. Archives of Internal Medicine. (1992) Purpose: A history of penicillin allergy does not predict reactions to subsequent administration of penicillin. This study tests the usefulness of skin tests with a major determinant and a minor determinant mixture in the prediction of IgE-mediated reactions. Methods: Eight centers cooperated in testing hospitalized adults with infectious diseases for which penicillin was the drug of choice. Infections were often the reason for admission but could have been hospital-acquired. Patients were tested with major and minor determinants followed by a therapeutic course of penicillin with observation for 48 hours. Results: There were 1539 patients who underwent skin testing (825 had histories of penicillin reaction – an astounding 54%, 104 were unsure of their status, and 610 had negative histories). No adverse reactions to the test materials were noted. One thousand, four-hundred and twentytwo patients completed the protocol. Among 726 history-positive patients, 566 with negative skin tests received penicillin and only seven (1.2%) had "possibly" IgE-mediated reactions. Among 600 history-negative patients, 568 with negative skin tests received penicillin and none had a reaction. Only nine of the 167 positive skin test reactors received penicillin, usually by "cautious incremental dosing". Two of these nine patients had reactions compatible with IgE-mediated or accelerated penicillin allergy and both were positive to two determinants. Conclusions: Negative skin tests continue to have a high negative predictive value and the reaction rate in skin-test positive patients was significantly higher. 3. ICU setting Arroliga M.E. et al. A pilot study of penicillin skin testing in patients with a history of penicillin allergy admitted to a medical ICU. Chest. (2000) Design: Prospective study of all patients with histories of penicillin allergy who were admitted to a medical ICU during a 3-month period and who received antibiotics at the Cleveland Clinic. Methods: Twenty-four patients were enrolled and 21 underwent skin testing (prick test and intradermal) with benzylpenicilloyl (major) and penicillin G (minor) along with histamine controls. Three patients were not skin tested due to recent history of Type I reaction to penicillin. Results: The characteristics of previous allergy were unknown in 11 patients, 6 had a history of rash, and 4 had a history of urticaria. Twenty of 21 patients had a negative skin test and a positive histamine control. One patient had a negative skin test and a negative histamine control but tolerated a penicillin challenge without problems. No adverse reactions to the skin test were noted and antibiotic coverage was changed in 10 patients (48%) as a result of the skin tests. Conclusions: A very small pilot study. The authors conclude that most patients without a history of Type I reactions have negative skin tests and are able to receive penicillin. Penicillin skin testing may be a way to alter antibiotic use patterns in ICU settings. 4. Safety – How often do patients have a systematic reaction to penicillin skin tests? Valyasevi M.A. et al. Frequency of systematic reactions to penicillin skin tests. Annals of Allergy, Asthma, and Immunology. (2000) Purpose: To determine the safety of penicillin skin testing and the rate of systemic reactions to skin testing in patients who gave a history of penicillin allergy. Methods: Retrospective chart review of 1710 patients with a history of penicillin allergy who underwent skin testing with major and minor (penicillin G) determinants (prick tests and intradermal). Results: Eighty-six patients had a positive skin test and 2 patients had systemic reaction. Neither patient required hospitalization and responded well to treatment with epinephrine in one case and chlortrimeton and prednisone in the other. The systemic reaction rate was 0.12% for all patients and 2.3% for the penicillin skin test positive group. No fatalities occurred. Conclusions: The incidence of systemic reaction to skin tests is low and skin prick tests should be performed. If patients have a history of a previous serious reaction, skin tests may be avoided and if done should be diluted. Practitioners who perform skin testing should be ready to treat a systemic reaction. IV. What are the potential antimicrobial resistance and economic benefits of clarifying penicillin allergy? In recent years, two very important issues have confronted medicine – antimicrobial resistance and the rising cost of health care. Penicillin skin testing may provide some relief for both of these problems. The following is a look at three recent studies that examine the issue. 1. Macy E. Elective penicillin skin testing and amoxicillin challenge: effect on outpatient antibiotic use, cost, and clinical outcomes. Journal of Allergy and Clinical Immunology. (1998) Purpose: To test the effect of penicillin skin testing on oral outpatient antibiotic use and to evaluate concerns over penicillin skin testing in advance of therapeutic penicillin use causing resensitization and increased drug allergy rates. Design: A natural history study on 236 patients who underwent penicillin skin testing with or without oral amoxicillin challenge between November 1994 and November 1995 to include those with both positive and negative skin tests. This was a follow-up to a study by Macy et al. published in 1997. That study tested a purified minor determinant mixture as part of a skin test protocol to see if it was predictive of penicillin allergy. Patients received the custom made skin tests followed by an oral challenge with amoxicillin if their skin tests were negative. Macy found that his purified minor determinant was safe and predictive of penicillin allergy. Methods: A review of computerized pharmacy records and outpatient medical records was undertaken with antibiotic prescriptions, clinic visit rates, and adverse reactions to antibiotics noted over a 2-year period to include the year prior to penicillin skin testing and the year after penicillin skin testing. Results: 1. In the follow-up period the use of penicillins increased from 13.7% to 39.8% of all antibiotics prescribed. 2. The overall use of antibiotics decreased from 779 courses dispensed to 191 patients to 558 courses dispensed to 169 patients. 3. Among patients with a negative skin test who received an antibiotic in the follow-up year, 47.4% received penicillin compared to 20.4% of those same patients who required antibiotics in the year prior to skin testing. 4. The total cost of antibiotics fell 32% ($17,211.88 to $11,648.27) during the year after skin tests. The average cost fell 7.2% (from $22.21 to $20.61) for patients with a negative skin test but rose 3.3% (from $21.61 to $22.34) for patients with a positive skin test. 5. The overall clinic visit rate did not change, a shift from primary care to specialty care visits was noted (51.9% to 39.1% to primary care). 6. Fifteen patients with positive skin tests received at least 1 course of a cephalosporin in the year after skin testing (33 total courses) with one reaction (rash in a one-year-old) noted that required no treatment. 7. Of the 93 patients with negative skin tests who subsequently received penicillin, 5 (5.4%) had an adverse reaction – all to amoxicillin. However, 2 of the 5 patients subsequently tolerated 5 additional courses of penicillin (including amoxicillin) and 1 patient reacted only after her 4th course to penicillin. All reactions were noted at least 7 days after the start of treatment. The adverse reaction rate in patients with a negative skin test and no amoxicillin rechallenge was 3.2% and the reaction rate to non-penicillins was 5.4%. Conclusions: Penicillin skin testing continues to be safe and changes subsequent frequency of antibiotic use and type of antibiotic prescribed. Cost was also decreased. Patients who received oral amoxicillin challenge with their initial skin testing were not resensitized with therapeutic penicillin use. In addition, those who had skin testing without oral amoxicillin challenge did not become sensitized when receiving penicillin. Also, cephalosporin use in penicillin allergic patients was safe. The fact that patients saw allergists at skin testing and more specialists in follow-up may have contributed to decreased antibiotic use by more accurate diagnosis and symptom relief. This may have confounded the study results. 2. Harris A.D. et al. Penicillin skin testing: a way to optimize antibiotic utilization. The American Journal of Medicine. (1999) Purpose: To test the hypothesis that performing penicillin skin testing as part of an antibiotic control strategy was feasible and could improve outcomes. Design: A pilot study of 100 consecutive patients seen over a 70 day period in 1997. Two groups of patients were included. Group one was the "therapeutic antibiotic group" and consisted of hospitalized patients who had a history of PCN allergy and required antibiotic treatment. Group two was the "prophylactic antibiotic group" and consisted of patients in a preadmission testing clinic before elective surgery who were to receive perioperative antibiotics. Patients with a history of b -lactam antibiotic-induced anaphylaxis, serum sickness, erythema multiforme, toxic epidermal necrolysis, use of antihistamines in the previous 72 hours, or those with antibiotic regimens that would not change regardless of the skin test results were excluded. Forty-four patients were identified. Methods: All patients received skin prick tests. If negative, intradermal tests with the PCN major determinant, the minor determinant, a negative control (diluent), and a positive control (histamine) were performed. After testing, the attending physician and house staff were notified of the results and if negative were given recommendations for antibiotic management with a b - lactam if appropriate. New treatment regimens were based on guidelines purposed by "published recommendations and ID specialists". Adverse events were assessed by daily follow-up and patients were contacted by telephone at least 2 weeks after discharge to assess subsequent adverse events. Antibiotic regimens ordered before skin testing were compared with antibiotics given after skin testing. Costs were calculated based on average wholesale drug prices. Results: Forty-four patients were enrolled with 28 in the therapeutic group and 16 in the prophylactic group. Twenty-four patients (55%) had a history of rash and 21/24 had negative skin tests. The other 20 patients had a history of either pruritus or GI symptoms or did not remember the details of their reaction and 17/20 had negative skin tests. Overall, 38/44 patients (86%) had negative skin tests, 3 patients (7%) had positive skin tests, and 3 patients (7%) had indeterminate skin tests. The recommendations to change antibiotics were followed in 36/44 patients. There were 23 patients in the therapeutic group whose antibiotics were changed. Prior to skin testing vancomycin was to be given in 11 patients, fluoroquinolones in 12 patients, and clindamycin in 10 patients. After skin testing, cephalosporins were given in 15 patients, penicillins in 7, and both in 1. All infections were cured. In the prophylactic group, 11/13 patients were to receive vancomycin and the other 2 were to receive ciprofloxacin and gentamicin. Instead, all received cefazolin and none developed postoperative infection. Vancomycin use was the most effected with 46 days of its use avoided. The projected reduction in the number of days of parenteral vancomycin use (based on the length of the study and the number of hospitalized patients using vancomycin) was 7%. The avoidance of cost was calculated for the therapeutic group (23 patients). The post skin test total cost for antibiotics was $2,440 as compared to the original regimens cost of $4,810. The yearly projected savings was about $12,400. No patient experienced adverse drug reactions. Conclusions: Although a small study, it suggests that penicillin skin testing can help reduce the cost of antibiotic therapy and the use of vancomycin in hospital settings without increasing the number of patients with adverse events or allergic reactions. 3. Perencevich EN et al. Benefits of negative penicillin skin test results persist during subsequent hospital admissions. Clinical Infectious Diseases. (2001) Purpose: To evaluate the effects of a negative initial penicillin skin test result on antibiotic use and possible adverse drug reactions during subsequent hospital admissions. Design: Thirty-eight patients who reported a penicillin allergy but subsequently had a negative skin test were analyzed. They were initially included in Harris et al.’s study mentioned above. After a 2-year period their charts were retrospectively reviewed to see the effect that a negative skin test had on antibiotics administered on subsequent hospital admissions. Methods: As described in the Harris et al. study, patients were initially tested with intradermal benzylpenicilloyl (major determinant) and a three part minor determinant mixture of sodium penicilloate, sodium penilloate, and sodium penicillin G. Their admissions over the next 2 years were reviewed and data concerning the antibiotics used, their duration, adverse events, infections treated, outcomes, and MRSA infections was collected. Results: The mean follow-up period was 575 days. For the 38 patients, there were 48 subsequent readmissions (14 patients) with antibiotics being prescribed in 35 of those admissions (73%). β-lactam use totaled 158 days. The frequency of antibiotic use is noted below and indication for therapy is noted below. All patients who were readmitted received a β-lactam during ≥ 1 admission. All infections were cured and no drug reactions to β-lactams were noted. All patients who received vancomycin had documented MRSA infection. Of note, all patients denied a penicillin allergy on admission. Antimicrobial agent # of patients Indications for antibiotics # of patients Cephalosporin 18 (51%) Bacteremia 1 Penicillin 14 (40%) Pneumonia 2 Vancomycin 14 (40%) UTI 4 Fluoroquinolone 9 (26%) Skin/soft tissue 27 Clindamycin 5 (14%) C. dif. Colitis 1 Gentamicin 3 (9%) Surgical prophylaxis 3 Metronidazole 2 (6%) Conclusions: 1. Suggests that the predictive power of negative skin test results is long-standing and reduces the concern that patients with negative skin tests could become resensitized. 2. Again, shows the profound effect on prescribing patterns in patients with negative skin tests including reducing the amount of vancomycin used and the cost of antimicrobial therapy. Suggests a "down- stream" effect on practice patterns. Study is limited by its small sample size and the retrospective data collection. Retrospective data collection precludes making judgments about what treatment patients would have received if still labeled with penicillin allergy. V. Brief comments on the use of cephalosporins – a relative contraindication if penicillin "allergic" It has become common practice by some physicians to avoid cephalosporins if a patient claims to be allergic to penicillin. In most situations this is unnecessary and again leads to the overuse of extended spectrum antibiotics. The common reactions to cephalosporins are listed below. The most common reaction is a maculopapular or morbilliform skin eruption. Penicillin-related compounds are produced by the cephalosporium mold and early cephalosporin antibiotics contained trace amounts of penicillins. This likely led to the over-estimation of cross-reactivity between penicillins and cephalosporins. The incidence of cross-reactivity for patients who are allergic to penicillin is now estimated to be between 6-8%. No skin test for cephalosporin allergy is currently available and desensitization exists but is not standardized. In Austrailia, assays for IgE antibodies to cephalosporins are in use. The clinical significance of these assays is unclear since the presence of IgE does not necessarily mean that patients will have an IgE-mediated reaction. Major (cephalosporoyl) and minor (cephalosporanyl) determinants may be used in skin testing in the near future. Type of Reaction Frequency Dermatologic 1.0-2.8% Positive direct antiglobulin test 1.0-2.0% Anaphylaxis 0.0001-0.1% Fever 0.5-0.9% Eosinophilia 2.7-8.2% (Kelkar et al.) There are three approaches to patients with penicillin allergy who could be given cephalosporins. (Kelkar et al.) 1. Avoid all β-lactams – a common approach that leads to increased cost and increased use of vancomycin. 2. Use common sense – take a thorough history and give the cephalosporin if patients previous reaction to penicillin was not life threatening. This is a good approach due to the extremely rare incidence of anaphylaxis and low likelihood that patient is allergic to penicillin. 3. Test patients with skin testing – test for penicillin allergy then give the cephalosporin if negative. This approach may be used in certain situations with patients who have a strong indication for cephalosporin use but a history of a serious penicillin reaction. VI. Clinical applications. For what infections would penicillin be preferred or absolutely indicated? In order for skin testing to be useful, there must be infections for which penicillin is equal to other antibiotics or absolutely indicated. The following is a list of potential situations in which penicillin would be absolutely indicated. All treponemal infections, particularly in pregnant patients. Pseudomonas pneumonias in seriously ill patients in which double-coverage with an antipseudomonal penicillin used in combination with a fluoroquinolone or aminoglycoside is needed. Neutropenic fever in which pseudomonas coverage is required. Several other infections for which penicillin is preferred have recently been studied and include: 1. MSSA pneumonia Gonzalez et al. (1999), looked at the clinical characteristics of patients with bacteremic pneumonias due to MRSA and MSSA. Surprisingly, they found that patients with MSSA bacteremic pneumonias (n=41 [41.5% treated with vancomycin and 24.4% treated with cloxacillin]) that were treated with vancomycin had a much higher mortality rate (47% to 0% p=<0.01) than those treated with cloxacillin. The groups appeared to be well matched. 2. Staphylococcal endocarditis Dodek et al. (1999) examined the issue of treating patients with a cloxacillin-sensitive Staphylococcus aureus endocarditis and a questionable history of immediate-type hypersensitivity to penicillin. They noted that in most cases, patients are treated with a b lactamase–resistant penicillins often with an aminoglycoside for the first few days. However, those with a penicillin allergy are often treated with vancomycin, which is more expensive and may be less effective than penicillin. These authors compared the utility of vancomycin versus cloxacillin in treating staph endocarditis and the cost of vancomycin versus the cost of the skin test. They found that there was a higher expected utility with cloxacillin and a lower cost associated with skin testing in most circumstances. They conclude that most patients with a history of immediate-type hypersensitivity should be skin tested before starting antimicrobial therapy. VII. Approaching the patient with penicillin allergy. How should clinicians approach this issue in a rational way so that penicillin is used in useful and appropriate ways? The following is a study that examined a practical approach to patients with penicillin allergy. Salkind A.R. et al. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA. (2001). Purpose: To determine the likelihood of true penicillin allergy with consideration of clinical history and to evaluate the diagnostic value added by appropriate skin testing. Methods: A meta-analysis of studies that described the accuracy or precision of skin testing in the diagnosis of IgE-mediated penicillin allergy by comparing the clinical history with the skin test result. Data from patients with "indeterminate" skin tests was excluded. Four studies were identified. Results: The presence of any clinical history suggesting penicillin allergy increases the likelihood that that patient will have a positive skin test (LR 1.9; 95% CI, 1.5-2.5). The absence of a history of penicillin allergy decreases the likelihood that that patient will have a positive skin test (LR 0.5; 95% CI, 0.4-0.6). Conclusions: Clinicians should systematically document signs and symptoms associated with the patient’s adverse reaction to penicillin. Most patients with a history of penicillin allergy can safely take penicillin if a combination of good history taking and skin testing is used. Suggestions for complete history taking are outlined below: Taking a History of Penicillin Allergy: What to Ask What was the patient’s age at the time of the reaction? Does the patient recall the reaction? If not, who informed them of it? How long after beginning penicillin did the reaction begin? What were the characteristics of the reaction? What was the route of administration? Why was the patient taking penicillin? What other medications was the patient taking? Why and when were they prescribed? What happened when the penicillin was discontinued? Has the patient taken antibiotics similar to penicillin (for example, amoxicillin, ampicillin, cephalosporins) before or after the reaction? If yes, what was the result? Practical Approach to Infectious Diseases by Richard E. Reese and Robert F. Betts suggests the following approach to patients with penicillin allergy and an infection. Their flow sheet provides a practical method for dealing with patients with penicillin allergy. Note that they recommend alternative therapies in the case of mild infections but promote good history taking and strategies for investigating penicillin allergy when serious infections are addressed. VIII. Conclusions: The vast majority of patients who claim to have penicillin allergy do not have an IgEmediated allergy and are unlikely to have any kind of reaction at all. Previous reactions may have been secondary to infection, other medications, or impurities in older preparations of penicillin. The avoidance of penicillin by physicians may be contributing to increasing antimicrobial resistance and health care costs. Penicillin skin testing is safe, predictive of penicillin allergy, and cost effective. Unfortunately, the availability of major and minor determinants limits its use at this time although many allergists still perform skin testing. Penicillin is the drug of choice for several types of infections and recent literature suggests it may be better than other agents (particularly vancomycin) in several infections. Cephalosporins rarely cause anaphylactic reactions and can be used safely in most patients including those with penicillin allergy. Avoiding cephalosporins should be considered if patients have a known IgE-mediated reaction to penicillins. A careful history can shed light on a claim of penicillin allergy that has been passed from physician to physician in the medical record. Physicians should not discount claims of penicillin allergy but instead take better histories and attempt to clarify the type of reaction. Physicians should only avoid penicillin and penicillin skin testing in patients with suspected or known IgE-mediated reactions. Patients with a previously held belief that they were allergic to penicillin seem to recall that they are not allergic after a negative skin test. Several "entry points" into the medical system such as pre-operative assessment clinics, STD clinics, and oncology clinics could be first to implement aggressive skin testing. This clarification of penicillin allergies could have a "trickle-down" effect, spreading into other areas of the medical community. Bibliography Kelkar PS, Li JT. Cephalosporin Allergy. The New England Journal of Medicine. 2001; 345(11): 804-809. Macy E. Elective penicillin skin testing and amoxicillin challenge: effect on outpatient antibiotic use, cost, and clinical outcomes. Journal of Allergy and Clinical Immunology. 1998; 102(2): 281285. Macy E, Richter PK, Falkoff R, Zeiger R. Skin testing with penicilloate and penilloate prepared by an improved method: amoxicillin oral challenge in patients with negative skin test responses to penicillin reagents. Journal of Allergy and Clinical Immunology. 1997;100(5):586-591. Dodek P, Phillips P. Questionable history of immediate-type hypersensitivity to penicillin in staphylococcal endocarditis: treatment based on skin-test results versus empirical alternative treatment-- a decision analysis. Clinical Infectious Diseases. 1999; 29: 1251-1256. Gonzalez C, Rubio M, Romero-Vivas J, Gonzalez M, Picazo JJ. Bacteremic pneumonia due to staphylococcus aureus: a comparison of disease caused by methicillin-resistant and methicillinsusceptible organisms. Clinical Infectious Diseases. 1999; 29: 1171-1177. Perencevich EN, Weller PF, Samore MH, Harris AD. Benefits of negative penicillin skin test results persist during subsequent hospital admissions. Clinical Infectious Diseases. 2001; 32: 317-319. Harris AD, Sauberman L, Kabbach L, Greineder DK, Samore MH. Penicillin skin testing: a way to optimize antibiotic utilization. The American Journal of Medicine. 1999; 107: 166-168. Gadde J, Spence M, Wheeler B, Adkinson NF. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA. 1993; 270(20): 2456-2463. Salkind AR, Cuddy PG, Foxworth JW. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA. 2001; 285(19): 2498-2505. Pichichero ME, Pichichero DM. Diagnosis of penicillin, amoxicillin, and cephalosporin allergy: reliability of examination assessed by skin testing and oral challenge. The Journal of Pediatrics. 1998; 132(1): 137-143. Valyasevi MA, Van Dellen RG. Frequency of systematic reactions to penicillin skin tests. Annals of Allergy, Asthma, and Immunology. 2000. 85: 363-365. Arroliga ME, Wagner W, Bobek MB, Hoffman-Hogg L, Gordon SM, Arroliga AC. A pilot study of penicillin skin testing in patients with a history of penicillin allergy admitted to a medical ICU. Chest. 2000; 118: 1106-1108. Sogn DD, Evans R, Shepherd GM, Casale TB, Condemi J, Greenberger PA, Kohler PF, Saxon A, Summers RJ, VanArsdel PP, Massicot JG, Blackwelder WC, Levine BB. Results of the National Institute of Allergy and Infectious Diseases collaborative clinical trial to test the predictive value of skin testing with major and minor penicillin derivatives in hospitalized adults. Archives of Internal Medicine. 1992; 152:1025-1032. Reese RE, Betts RF. A Practical Approach to Infectious Diseases 4 th edition. Little, Brown and Company: New York. 1996. Middleton E, Ellis EF, Yunginger JW, Reed CE, Adkinson FN, Busse WW. Allergy: Principles and Practice. Mosby. Baltimore. 1998. Kniker WT, Hales SW, Lee LK. Diagnostic methods to demonstrate IgE antibodies: skin testing techniques. Bulletin of the New York Academy of Medicine. 1981:57(7):524-546. Mangi RJ. Allergy skin tests: an overview. Otolaryngologic Clinics of North America. 1985:18(4): 719-723.