Department of Pharmacy
advertisement

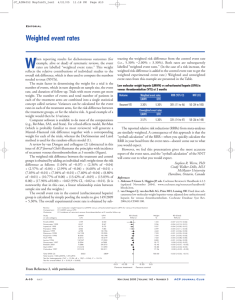
OUMC Anticoagulation Team Clinical Guidelines – Low Molecular Weight Heparin Updated: 3/04/2009 As a means of reducing the likelihood of patient harm associated with the use of anticoagulation therapy within OUMC, the OUMC Anticoagulation Team has developed the following Clinical Guidelines for use of low molecular weight heparin within OU Medical Center. Clinical Guidelines – Low Molecular Weight Heparin Responsible Party Pharmacy and Therapeutics Committee Medical Staff Pharmacy Nursing D:\106737052.doc 1. Reviews all adverse drug reactions associated with low molecular weight heparin (LMWH) therapy and initiates appropriate follow-up actions. 2. Ensures that the LMWH antidote, protamine sulfate, remains on the formulary and is available for use. 3. Develops MUE criteria for subsequent review/reporting through the Joint Quality Review and Medical Executive Committees. 1. 2. 3. 4. Initiates either written or electronic (ePOM) orders for LMWH. Specifies the indication requiring anticoagulation therapy in the orders or medical record. Orders a serum creatinine (SCr) level within 24 hours before initiation of therapy for patients with renal compromise. Monitors platelet count every two-three days while hospitalized. 1. Ensures that only manufacturer’s unit-dose syringes are procured whenever available; otherwise, unit-dose and barcoded packaging is provided before LMWH is dispensed. 2. References medical staff approved dose rounding chart for enoxaparin and modifies ordered dose in a manner that allows dispensing of unit-dose syringes. (Refer to Pharmacy P&P 13-15). 3. Schedules daily doses of LMWH to be given at 0600 daily on eMAR or as otherwise noted by prescriber. 4. Reviews Scr prior to initial order entry/validation and upon subsequent changes in the medication regimen. 5. Dispenses initial LMWH dose and dose modifications only if related lab results are appropriate. 6. Evaluates the potential for drug-drug interactions involving LMWH. 7. Initiates clinical interventions with the prescriber for any deviations from these guidelines. 8. Maintains the LMWH antidote, protamine sulfate, in the pharmacy at all times. 1. Administers daily doses of LMWH at 0600 hours or as otherwise ordered by the prescriber. 2. When giving enoxaparin injection: a.) Do not expel the air bubble from the 30 mg or 60 mg syringe before injection. Clinical Guidelines – Low Molecular Weight Heparin Responsible Party 3. 4. 5. 6. 7. Laboratory/Blood Bank Services b.) Do not administer intramuscularly. c.) Alternate between the left and right anterolateral and left and right posterolateral abdominal wall. d.) Introduce the whole length of the needle at a 45 to 90 degree angle into a skin fold held between the thumb and forefinger. Hold the skin fold throughout the injection. e.) Do not rub the injection site after completion of the injection. Alerts the physician for signs/symptoms of bleeding or thrombosis, or falls. Limits the use of intramuscular injections of concomitant medications to the upper extremities for patients receiving LMWH therapy. Provides education to the patient and family using OU MEDICAL CENTER Patient Education Committee-approved material. May initiate a pharmacy consult if additional education is needed. At discharge, reviews with patient/family members the follow-up instructions from the physician relative to anticoagulation medications, e.g. follow-up appointment information. 1. Maintains Fresh Frozen Plasma (FFP) at all times for the emergent management of bleeding and excessive anticoagulation. Approvals: OU Medical Center Anticoagulation Team: OU Medical Center Medication Management Team: OU Medical Center Pharmacy and Therapeutics Committee: OU Medical Center Joint Quality Review Committee: OU Medical Center Medical Executive Committee: D:\106737052.doc