Chapter 9 – Solution Chemistry
advertisement
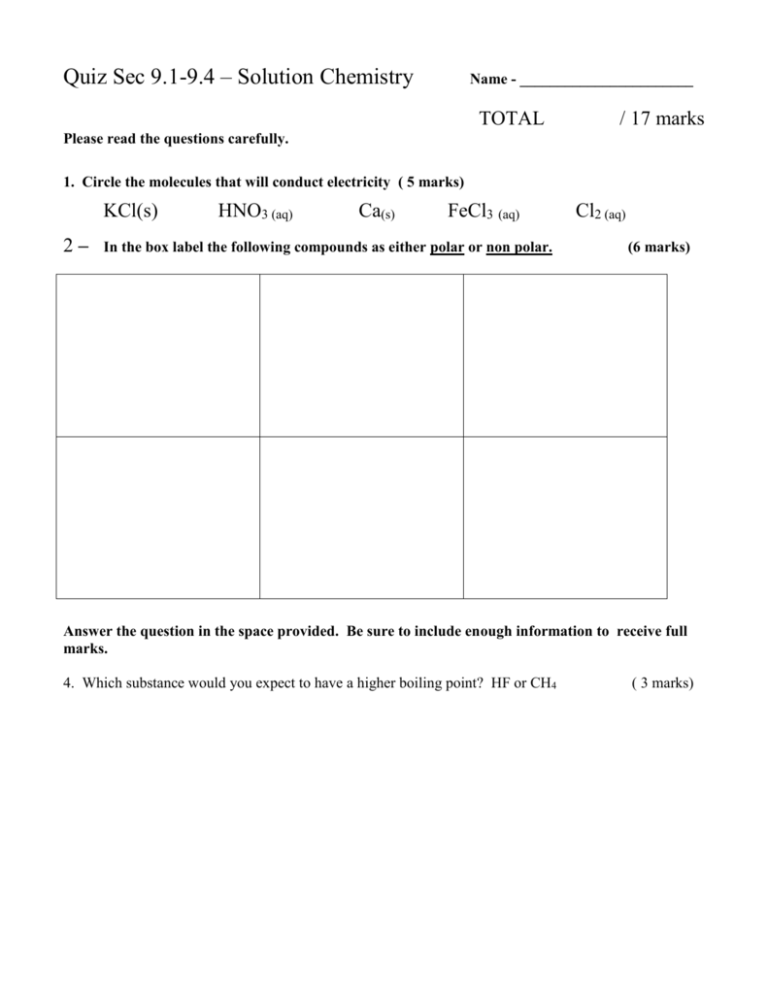
Quiz Sec 9.1-9.4 – Solution Chemistry Name - _______________________ TOTAL / 17 marks Please read the questions carefully. 1. Circle the molecules that will conduct electricity ( 5 marks) KCl(s) 2– HNO3 (aq) Ca(s) FeCl3 (aq) In the box label the following compounds as either polar or non polar. Cl2 (aq) (6 marks) Answer the question in the space provided. Be sure to include enough information to receive full marks. 4. Which substance would you expect to have a higher boiling point? HF or CH4 ( 3 marks) 5. What would be a better solvent to dissolve KCl, butane ( CH3CH2CH2CH3) or compound X ( O-C-Cl-CH2CH2COOH) ? Be specific in your answer. (3 marks)











