Synthesis of Reference Compounds
advertisement

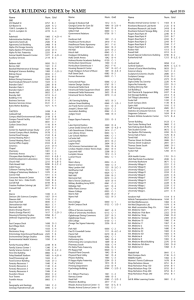
Supplementary Information Wool 200 – 400 mg Headspace Analysis Extraction 70 mg ca. 400 mg Enrichment with SEP50 1 h @ 70 C Closed-loop stripping plus enrichment with SEP50 1 h @ 70 C DCM Extraction 1 ml TBME Extraction 1 ml Fig. S1. Wool sample allocation for gas chromatography-mass spectrometry analysis Fig.S2. Diapered and wrapped alien lamb dressed in a cotton fleece jacket worn for 24 hr by the ewe’s own lamb. 0.002 0.000 0.001 Density 0.003 0.004 density.default(x = uit[[2]][, 2]) 1000 1200 1400 1600 1800 2000 2200 2400 Value of test statistic N = 10000 Bandwidth = 15.95 Fig. S3. Permutation distribution of the test statistic showing the differences in the composition of the wool volatiles collected from day-old twin lambs compared to the rest of the group of lambs. The calculated P-value < 0.001 is indicated by the vertical line on the right hand side of the figure. Twin lambs thus possess odor profiles that are unique and their odor profiles are more similar than those of other randomly selected non-twin lambs. Synthesis of Reference Compounds. Some of the compounds that were required as standards for the confirmation of the structures of constituents identified in this study were available from previous studies in our laboratory, or from the South African distributors of the products of Sigma-Aldrich, Merck, Saarchem, NT Laboratories and B.D.H.; others were synthesized from commercially available starting materials as described below. The structures of all of the compounds that were synthesised in the present investigation were verified by GC-MS, 1 H and 13C NMR spectrometry. Some of the synthesized compounds belong to homologous series of the respective long-chain compounds. The NMR spectra of compounds belonging to these homologous series are practically identical and the 13C NMR data of only one example of each compound class are included in the information below. Preparation of 2,6,10,14-tetramethylhexadecane. A solution of (6E,10E)-7,11,15trimethyl-3-methylenehexadeca-1,6,10,14-tetraene (Burger et al., 1978) (0.25 g, 0.92 mmol) in glacial acetic acid (50 ml) was hydrogenated using platinum on activated charcoal (10% Pt/C) as catalyst. The hydrogenation was carried out at approximately atmospheric pressure and the consumption of hydrogen was monitored volumetrically. The reaction was allowed to run to completion, the reaction mixture diluted with an equal volume of distilled water, and then extracted with CHCl3 (3 x 10 ml). The combined extracts were washed with water to neutral pH and dried on anhydrous MgSO4, after which the drying agent was filtered off and the solvent removed on a rotary evaporator to yield 2,6,10,14-tetramethylhexadecane in quantitative yield with a purity of 91% (GC-MS). MS (70 eV): m/z (%) 282(M+, 0.07), 267(0.13), 253(0.17), 197(3), 183(4), 155(3), 141(5), 127(7), 113(8), 99(14), 85(44), 71(84), 57(100), 43(52). 13C NMR (CDCl3, 101 MHz): δ = 11.45 (q, C-16), 19.26 (q, C-20)3, 19.32 (q, C-19*)4, 19.81 (q, C-18*), 22.68 (q, C-17*), 22.77 (q, C-1*), 24.54 (t, C-8*), 24.55 (t, C-12*), 24.86 (t, C-4*), 28.04 (d, C-2), 29.63 (t, C-15), 32.83 (d, C-6*), 32.87 (d, C-10*), 34.49 (d, C-14), 37.01 (t), 37.35 (t), 37.45 (t), 37.46 (t), 37.52 (t), 39.44 (t, C-3). Note. * exchangeable assignments Preparation of branched primary alcohols. Primary isoalcohols and ante-isoalcohols were synthesized from the corresponding branched carboxylic acids according to a scaled-down version of the protocol of Tietze and Eicher (1981: 416), as exemplified by the synthesis of 15-methyl-1-hexadecanol. The resulting alcohols were used as starting materials for the preparation of the branched aldehydes described below. Note. Methyl substituents are numbered in order of their attachment to carbon atoms with ascending numbers in the parent-chain. 15-Methyl-1-hexadecanol. 15-Methylhexadecanoic acid (8.00 mg; 0.03 mmol) in TBME (200 μl) was placed in a 2 ml Reacti-Vial, with a small-vaned magnetic follower, and treated with an excess of a clear standardized solution of LiAlH4 in dry diethyl ether. The Reacti-Vial was sealed and the reaction mixture stirred at 60°C for 3 hr. The excess LiAlH4 was decomposed by the dropwise addition of water (500 μl) with stirring. The white precipitate was dissolved by the addition of a few drops of conc. H2SO4 (350 μl). The organic phase was separated from the aqueous phase and the aqueous phase extracted with TBME (100 μl). The combined extracts were washed to neutral pH with water and dried on anhydrous MgSO4. The drying agent was filtered off and the solvent removed on a rotary evaporator to yield 15-methyl-1-hexadecanol (6.00 mg, 79%), with a purity of 95% (GC-MS). MS (70 eV): m/z (%) 238(1.3), 210(4), 182(4), 111(25), 97(42), 83(60), 69(78), 57(87), 55(86), 43(100), 41(74). 8-Methyl-1-nonanol MS (70 eV): m/z (%) 140(M+, 4), 126(14), 112(11), 111(17), 97(14), 85(50), 83(30), 71(51), 70(52), 69(79), 57(74), 56(58), 55(65), 43(100), 41(84). 11-Methyl-1-tridecanol. MS (70 eV): m/z (%) 196(0.04), 168(1.5), 166(2), 139(4), 125(25), 97(50), 83(72), 71(80), 69(100), 57(85), 55(94), 43(50), 41(62). 12-Methyl-1-tridecanol. MS (70 eV): m/z (%) 196(0.5), 168(4), 140(6), 125(4), 111(7), 97(33), 83(56), 69(84), 57(84), 56(100), 55(88), 43(93), 41(81). 13-Methyl-1-tetradecanol. MS (70 eV): m/z (%) 210(0.02), 140(0.2), 125(3), 111(4), 97(18), 83(32), 69(100), 57(33), 56(92), 55(95), 43(72), 41(62). 14-Methyl-1-pentadecanol. MS (70 eV): m/z (%) 224(0.8), 196(4), 168(5), 140(4), 125(6), 111(21), 97(39), 83(59), 69(81), 57(86), 56(86), 55(84), 43(100), 41(78). 14-Methyl-1-hexadecanol. MS (70 eV): m/z (%) 238(0.5), 210(3), 125(13), 111(30), 97(65), 83(79), 70(98), 57(92), 55(100), 43(72), 41(88). Preparation 2-pentadecanol. Following the protocol of Brown and Geoghegan (1967), 1-pentadecene (4.80 g, 0.022 mol) was added to a solution of mercuric acetate (7.01 g, 0.022 mol), in water (22 ml) and purified tetrahydrofuran (22 ml), in a 150-ml roundbottom flask containing a magnetic follower (stir bar). The reaction mixture was stirred for 1.5 hr at room temperature to complete the oxymercuration stage. Sodium hydroxide solution (20 ml, 3 M) was then added, followed by a solution of sodium borohydride (20 ml, 0.5 M) in sodium hydroxide (3.0 M). The mercury was allowed to settle and the reaction mixture diluted with purified diethyl ether (20 ml). The organic phase was separated from the aqueous phase and dried on anhydrous MgSO4. The drying agent was filtered off, and the solvent removed on a rotary evaporator to yield 2-pentadecanol (2.88 g, 57%), with a purity of 82% (GC-MS). MS (70 eV): m/z (%) 210(2), 125(6), 111(8), 97(15), 83(12), 69(12), 57(20), 55(23), 45(100), 43(41). This product was used for the preparation of 2-pentadecanone as described below. Preparation of 6,10,14-trimethyl-2-pentadecanol. 6,10,14-Trimethyl-2-pentadecanone (hexahydrofarnesyl acetone) (50 μl, 0.15 mmol) in TBME (100 μl) was placed in a 2 ml Reacti-Vial with a magnetic follower and reduced with LiAlH4, as described above for the primary alcohols, to yield 6,10,14-trimethyl-2-pentadecanol (31.87 mg, 91%), with a purity of 93% (GC-MS). MS (70 eV): m/z (%) 210(0.3), 196(1), 182(2), 167(1), 140(3), 125(17), 111(24), 97(44), 83(25), 71(63), 69(60), 57(100), 55(70), 45(69), 43(61), 41(38). 13C NMR (CDCl3, 101 MHz): δ = 19.70 (q, C-16), 19.77 (q, C-17), 22.64 (q, C18*), 22.74 (q, C-15*), 23.44 (t, C-8), 23.53 (q, C-1), 24.68 (t, C-4*), 24.83 (t, C-12*), 28.00 (d, C-14), 32.79 (d, C-10*), 32.81 (d, C-6*), 37.05 (t, C-5*), 37.15 (t, C-7*), 37.30 (t, C-9*), 37.41 (t, C-11*), 39.39 (t, C-13*), 39.75 (t, C-3*), 68.19 (d, C-2). Preparation of aldehydes. Branched and unbranched aldehydes were synthesized by oxidation of the corresponding primary alcohols with pyridinium chlorochromate (PCC), according to Harwood et al. (2003), in yields ranging from 53 to 84%, as exemplified by a microscale preparation of 15-methylhexadecanal from 15-methyl-1-hexadecanol. 15-Methylhexadecanal. 15-Methyl-1-hexadecanol (6.00 mg, 0.023 mmol), dissolved in DCM (1 ml) was added dropwise to a magnetically stirred suspension of PCC (7.58 mg, 0.352 mmol) in DCM (200 μl) at 0ºC. The reaction mixture was stirred at room temperature for 2 h, diluted with dry diethyl ether (50 μl) and the supernatant solution decanted from the black residue. The residue was washed with diethyl ether (3 x 30 μl), employing centrifugal separation of the phases, and the combined ether and DCM phases filtered through a short column of silica gel. The solution was dried on anhydrous MgSO4, then the drying agent filtered off and the solvent removed on a rotary evaporator to yield 15-methylhexadecanal (5.00 mg, 84%), with a purity of 98% (GC-MS). MS (70 eV): m/z (%) 254(M+, 0.1), 236(0.3), 210(1), 208(2), 180(2), 154(2), 152(3), 137(4), 123(8), 109(14), 96(23), 95(31), 82(40), 81(43), 69(61), 67(47), 57(100), 55(71), 43(91), 41(62). 13C NMR (CDCl3, 150.88 MHz): δ = 22.70 (q, C-16, C-17), 28.01 (d, C-15), 29.10 (t), 29.21 (t), 29.27 (t), 29.39 (t), 29.47 (t), 29.63 (t), 29.67 (t), 29.70 (t), 29.75 (t), 29.98 (t), 33.82 (t, C-3), 39.10 (t, C-14), 43.96 (t, C-2), 203.03 (d, C-1). 3-Methylpentanal. MS (70 eV): m/z (%) 100(M+, 6), 82(2), 71(19), 58(39), 57(50), 56(100), 44(55), 43(79), 41(86). 13C NMR (CDCl3, 75.38 MHz): δ = 11.23 (q, C-5), 19.45 (q, C-6), 29.69 (t, C-4), 31.68 (d, C-3), 41.15 (t, C-2), 203.21 (d, C-1). 8-Methylnonanal. MS (70 eV): m/z (%) 138(0.4), 128(4), 114(6), 110(7), 95(34), 82(45), 81(31), 71(31), 69(45), 67(27), 57(93), 55(56), (100), 41(95). 11-Methyltridecanal. MS (70 eV): m/z (%) 212(M+, 0.1), 194(0.4), 184(0.7), 183(1), 166(2), 165(3), 139(3), 138(2), 137(5), 123(6), 109(26), 96(28), 95(46), 83(42), 82(38), 81(40), 70(59), 69(40), 67(36), 57(95), 55(78), 43(67), 41(100). 12-Methyltridecanal. MS (70 eV): m/z (%) 212(M+, 0.1), 194(0.4), 184(1), 168(2), 166(2), 153(2), 151(2), 140(3), 138(3), 137(4), 123(7), 110(8), 109(16), 96(20), 95(37), 83(40), 81(47), 71(35), 69(61), 67(45), 57(100), 55(74), 43(92), 41(67). 13-Methyltetradecanal. MS (70 eV): m/z (%) 208(2), 182(3), 180(2), 154(2), 152(2), 137(3), 124(6), 123(6), 109(12), 96(26), 95(28), 82(46), 71(25), 69(43), 68(31), 67(31), 57(79), 55(60), 43(100), 41(83). 14-Methylpentadecanal. MS (70 eV): m/z (%) 222(1), 212(1), 196(1), 194(2), 166(2), 164(2), 152(2), 138(3), 137(4), 124(5), 123(6), 109(14), 96(28), 95(30), 83(31), 82(51), 81(30), 69(43), 57(79), 55(70), 43(100), 41(81). 14-Methylhexadecanal. MS (70 eV): m/z (%) 236(1), 225(1), 208(1), 181(1), 151(2), 137(5), 123(9), 109(21), 96(30), 95(41), 82(45), 81(45), 70(64), 69(49), 67(35), 57(100), 55(79), 43(77), 41(92). 13C NMR (CDCl3, 150.88 MHz): δ = 11.44 (q, C-16), 19.26 (q, C-17), 24.73 (t, C-12), 29.10 (t, C-11), 29.27 (t), 29.53 (t, 2 x C), 29.62 (t), 29.67 (t), 29.70 (t), 29.75 (t), 30.06 (t), 31.96 (t, C-3), 34.44 (d, C-14), 36.68 (t, C-13), 43.95 (t, C2), 203.00 (d, C-1). Tetradecanal. MS (70 eV): m/z (%) 212(M+, 0.13), 194(1), 184(1), 168(5), 166(3), 138(7), 124(9), 110(38), 96(32), 82(38), 69(27), 68(28), 67(28), 57(82), 55(77), 43(100), 41(95). 13C NMR (CDCl3, 101 MHz): δ = 14.11 (q, C-14), 22.11 (t, C-13), 22.71 (t, C12), 29.09 (t), 29.19 (t), 29.27 (t), 29.38 (t), 29.45 (t), 29.61 (t), 29.67 (t), 29.69 (t), 31.94 (t, C-3), 43.92 (t, C-2), 203.00 (d, C-1). Pentadecanal. MS (70 eV): m/z (%) 226(M+, 0.2), 208(2), 182(4), 180(3), 152(5), 138(6), 124(8), 110(14), 96(40), 82(44), 69(22), 68(23), 67(22), 57(65), 55(61), 43(93), 41(100). Hexadecanal. MS (70 eV): m/z (%) 240(M+, 0.2), 222(2), 194(3), 166(3), 152(3), 138(6), 124(10), 110(16), 109(15), 96(45), 82(53), 69(25), 68(26), 67(25), 57(61), 55(58), 43(100), 41(94). Heptadecanal. MS (70 eV): m/z (%) 254(M+, 0.2), 236(2), 210(3), 208(3), 180(2), 152(3), 138(6), 137(5), 124(10), 123(8), 110(15), 109(14), 96(43), 82(47), 69(23), 68(23), 67(22), 57(60), 55(57), 43(100), 41(85). Preparation of methyl ketones. Methylketones were prepared by PCC oxidation, according to Harwood et al. (2003), from the corresponding secondary alcohols, as described in the previous section. 2-Tetradecanone. MS (70 eV): m/z (%) 212(M+, 4), 197(2), 154(4), 153(3), 127(2), 111(2), 110(2), 96(6), 85(11), 71(42), 59(48), 58(100), 55(18), 43(96), 41(33). 13C NMR (CDCl3, 75.38 MHz): δ = 14.10 (q, C-14), 22.68 (t, C-13), 23.88 (t, C-4), 29.18 (t), 29.35 (t), 29.40 (t), 29.47 (t), 29.53 (t), 29.61 (t), 29.65 (q, C-1), 29.81 (t), 31.92 (t, C-12), 43.81 (t, C-3), 209.30 (d, C-2). 2-Pentadecanone. MS (70 eV): m/z (%) 226(M+, 7), 211(3), 168(6), 138(3), 127(5), 110(5), 96(11), 85(17), 71(39), 59(68), 58(92), 43(100), 41(55). Preparation of 6,10-dimethyl-2-undecanone. 6,10-Dimethyl-5,9-undecadien-2-one (geranylacetone) (0.25 g, 1.29 mmol) was hydrogenated in glacial acetic acid (50 ml) using platinum on activated charcoal (10% Pt/C) as catalyst at atmospheric pressure. The consumption of hydrogen was monitored and was terminated after the consumption of a volume of hydrogen equivalent to the reduction of two double bonds. The reaction mixture was then diluted with an equal volume of distilled water and extracted with CHCl3 (3 x 10 ml). The combined extracts were washed with water to neutral pH and dried on anhydrous MgSO4, after which the drying agent was filtered off and the solvent removed on a rotary evaporator to yield 6,10-dimethyl-2-undecanone (0.24 g, 95%), with a purity of 88% (GC-MS). MS (70 eV): m/z (%) 198(M+, 0.4), 180(4), 140(3), 123(3), 109(11), 95(11), 85(16), 71(28), 58(77), 43(100), 41(23). 13C NMR (CDCl3, 100.58 MHz): δ = 19.55 (q, C-12), 21.44 (t, C-4), 22.69 (q, 2 x C), 24.73 (t, C-8), 27.96 (d, C10), 29.83 (q, C-1), 32.65 (d, C-6), 36.52 (t, C-7), 37.10 (t, C-5), 39.32 (t, C-9), 44.14 (t, C-3), 209.35 (d, C-2). References 1. Brown, H. C. and Geoghegan, P. 1976. The oxymercuration-demercuration of representative olefins. A convenient, mild procedure for the Markovnikov hydration of the carbon-carbon double bond. J. Am. Chem. Soc. 89: 1522–1524. 2. Burger, B. V., Le Roux, M., Spies, H. S. C., Truter, V. and Bigalke, R. C. 1978. Mammalian pheromone studies, III. (E,E)-7,11,15-Trimethyl-3methylenehexadeca-1,6,10,14-tetraene, a new diterpene analogue of b-farnesene from the dorsal gland of the springbok, Antidorcas marsupialis. Tetrahedron Lett. 52: 5221–5224. 3. Harwood, L. M., Moody, C. J. and Percy, J. M. 2003. Experimental organic chemistry. Standard and microscale. 2nd ed, Blackwell Science, Germany. Experiment 31, pp 507–510. 4. Tietze, L.-F. and Eicher, T. 1981. Reaktionen und Synthesen im organishchemischen Praktikum. Georg Thieme, Stuttgart, Germany.