Chapter 15 Sample Problems
advertisement

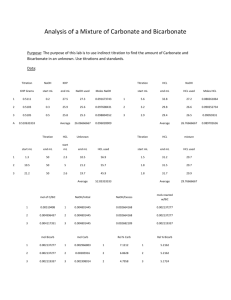
Chapter 15 Sample Problems 1. Calculate the [H+] in a solution containing 1.00 M HF (Ka = 7.2 x 10-4) and 0.50 M NaF. 2. A solution contains 0.050 M HA and 0.025 M NaA and has a pH of 4.0. Calculate the Ka value for HA. 3. Consider a solution that contains 0.10 M acetic acid (Ka = 1.8 x 10-5) and 0.10 M sodium acetate. a. What is the pH of this solution? b. Calculate the pH of this solution if 0.010 mol of NaOH is added to 1.0 L of this buffered solution. c. Contrast this pH change with the one that occurs with 0.010 mol of NaOH is added to 1.0 L of pure water. 4. Consider a solution containing 0.30 M NH3 and 0.20 M NH4Cl. a. What is the pH of this solution? b. Calculate the pH after 0.050 mol of HCl is added to this solution. c. Calculate the pH after 0.030 mol of NaOH(s) is added to the original solution. 5. A solution contains 0.25 M HOCl and 0.75 M NaOCl. Calculate the pH of this solution. (Ka = 3.5 x 10-8) 6. Use the Henderson-Hasselbach equation to solve sample problem #4a. 7. A chemist who wishes to prepare a solution buffered at pH = 4.0 can choose among the following acids: Weak acid Ka ]HA 1.0 x 10-2 HB 1.0 x 10-4 HC 1.0 x 10-6 Which acid (along with its conjugate base) should the chemist choose? 8. Consider the titration of 100.0 mL of 1.00 M HCl with 0.500 M NaOH. Calculate the [H+] in the solution after 50.0 mL of 0.50 M NaOH has been added. 9. Consider the titration of 50.0 mL of 0.200 M HNO3 with 0.100 M NaOH. Calculate the pH at the following points in the titration: Total volume of 0.100 M NaOH added (mL) A. 0 B. 10.0 C. 20.0 10. Consider the titration of 50.0 mL of 0.10 M acetic acid with 0.10 M NaOH. (Ka for acetic acid is 1.8 x 10-5) Calculate the pH at each of the following total added volumes of NaOH. A. 0 mL B. 10.0 mL of 0.10 M NaOH added C. 25.0 mL of 0.10 M NaOH added to the original solution D. 40.0 mL (total) of NaOH added E. 50.0 mL (total) of NaOH added F. 60.0 mL (total) of NaOH added G. 75.0 mL (total) of NaOH added 11. A 0.350 g sample of a solid weak acid is dissolved in 50.0 mL of water and titrated with 0.100 M NaOH. A. 23.2 mL of 0.100 M NaOH is required to reach the equivalence point. What is the molecular weight of the acid? B. After 10.0 mL of 0.100 M NaOH was added, the pH was measured and found to be 4.00. Calculate the Ka for the weak acid. 12. An indicator, HIn (Ka = 1.0 x 10-6) where HIn is red and In- is blue, is placed in a solution of a strong acid. The solution is then titrated with a NaOH solution. At what pH will the indicator color change occur? 13. Consider the titration of 100.0 mL of 0.100 M HCl with 0.1000 M NaOH. What should be the Ka value of the indicator used for this titration? 14. Consider the titration of 50.00 mL of 0.500 M HOCl (Ka = 3.50 x 10-8) with 0.100 M NaOH. What value of Ka should an indicator have to be used to mark the equivalence point of this titration? 15. An indicator, HIn, has a Ka value of 1.0 x 10-7. Determine at which pH the color change occurs when the titration is used to mark the equivalence point for A. the titration of an acid. B. the titration of a base.