Week 8_overview
advertisement

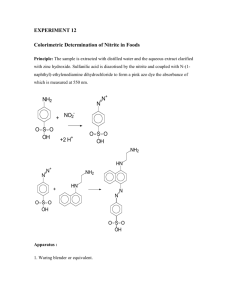
Week 8 Studio 8a AQ1: nitrate See Table 2 F_06_S8a_AQ1_nitrate Napthylamine Hydrochloride Reagent: (need 20 mL per student group) Dissolve 1 mL concentrated HCl in 50 mL deionized water. Then dissolve 0.60 grams 1-napthylamine in the solution and stir for 15 minutes. Transfer to 100 mL volumetric flask and dilute to the mark. Store in a refrigerator or ice bath in a brown bottle; the solution is stable for 1 week. Possibly make nitrate standard IS 8 Studio 8b Precipitation Reactions Aqueous case study Studio 8c AQ2: chloride Clear well plates 8 dropper bottles of 0.1M solutions of NH4NO3 KNO3 Ca(NO3)2 Sr(NO3)2 Mg(NO3)2 Al(NO3)3 Fe(NO3)3 NaCl NaClO4 NaOH Na2CO3 Na2SO4 Na3PO4 1 dropper bottle of 0.1M AgNO3 (perform demo of AgNO3 + NaCl note coursepack says Ag3PO4) See Table 1 F_06_S8b_precipitation F_06_S8c_AQ2_chloride Table 2: Supplies for Week 6, Lab 2 (Amounts per section?) Chemicals Supplies Needed Supplies we have Concentrated 2 1- 500mL amber HCl 7647-01-0 mL bottle 2.4 M 140 mL 12-plastic bottles HCl 7647-01-0 that will hold 100 mL Finely powdered 6-10 mL glass vials zinc 7440-66-6 1g with caps 1-napthylamine 1.2 g 134-32-7 potassium nitrate 3g 7757-79-1 sodium acetate 0.20 M 140 mL 127-09-3 sodium chloride 200g 7647-14-5 sulfanilic acid (4- 0.03464 M* 140 (H2H)C6H4SO3H) mL 121-57-3 173.19 g/mol Unknown *Dissolve 0.60 g dry dulfanilic acid in 70 mL hot water dilute to 100 mL in volumetric flask Napthylamine Hydrochloride Reagent: (need 20 mL per student group) Dissolve 1 mL concentrated HCl in 50 mL deionized water. Then dissolve 0.60 grams 1-napthylamine in the solution and stir for 15 minutes. Transfer to 100 mL volumetric flask and dilute to the mark. Store in a refrigerator or ice bath in a brown bottle; the solution is stable for 1 week. Zinc NaCl Mixture: (need about 20 g per student group) Mix 200 grams of granular NaCl with 1.00 gram of finely powdered Zinc. Mix the two powders thoroughly by shaking in a flask for several minutes. Stock Potassium Nitrate Solution: Stock A: (500 ppm NO3-) Dry 2-3 grams KNO3 at 120C for 1 hour. Cool in a desicator. Dissolve 0.816 grams of the dried KNO3 in 500 mL deionized water and dilute to 1 L. Stock B: (100 ppm NO3-) Pipet out 50 mL of Stock A solution and dilute to a volume of 250 mL in a volumetric flask to produce the stock 100 mg/L standard that will be used throughout the experiment. Need 100-150 mL per student group. Hydrochloric Acid Reagent: (need 20 mL per student group) Dilute 100 mL concentrated HCl to 500 mL with water and mix thoroughly. Sulfanilic Acid Solution: (need 20 mL per student group) Dissolve 0.60 grams dry sulfanilic acid in about 70 mL of hot water. Once dissolved, dilute the sulfanilic acid solution to 100 mL in a volumetric flask and mix thoroughly. Sodium Acetate Solution: (need 20 mL per student group) Prepare 100 mL of sodium acetate solution by dissolving 16.4 grams of sodium acetate in 5 mL deionized water and diluting to 100 mL mark with DI water. Well Water Unknown: (Conc: 50 ppm NO3-, need 50 mL per student group). Add 10 mL of the KNO3 Stock A solution (500 ppm) to a 100-mL volumetric flask, dilute to the mark with H2O. Students prepare a primary standard solution of NaCl and use it to standardize the AgNO3. Then they test for chloride ion concentration through a precipitation titration with potassium chromate as the indicator. Table 1: Week 7, lab 2 chemicals needed Chemicals 1.9 L (0.0025M) this is a standard solution so accuracy is 7761-88-8 very important. (in at least 2 bottles) K2CrO4 140 mL (0.014M) (in 4 bottles) 7789-00-6 Unknown 1.5 L “septic 55 ppm Cl-** system effluent” Reagents per 24 student section ** Add 0.9068 g dried primary standard NaCl to a 100 mL volumetric flask and dilute to the mark. Pipet 10 mL of this solution into a 1.00 L volumetric, dilute to the mark with DI water. This solution doesn’t have to be exact, but in the range of 55 ppm. +/- 10 ppm. AgNO3