doc
advertisement

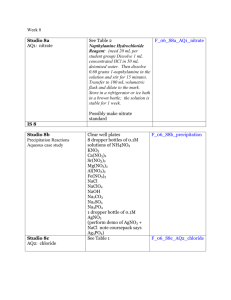
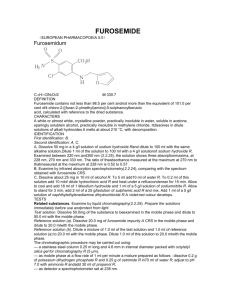
DETERMINATION OF NITRITE-N IN WATER APPARATUS: Spectrophotometer for use at 543 nm, providing a light path of I cm or longer REAGENTS: Nitrite free water: Use nitrite free water in making all reagents and dilutions Color reagent: To 800 ml water, add 100 ml 85 % phosphoric acid and 10 g sulfanilamide. After dissolving sulfanilamide completely, add 1 g N-(1-naphthyl)ethylenediamine dihydrochloride. Mix to dissolve, then dilute to 1 liter with water. Solution is stable for about a month when stored in a dark bottle in refrigerator. Sodium oxalate, 0.025M (0.05N): Dissolve 3.350 g Na2C2O4, primary standard grade, in water and dilute to 1000 ml. Ferrous ammonium sulfate, 0.05M (0.05N): Dissolve 19.607 g Fe(NH4)2 (SO4)2 .6H2O plus 20 ml of conc. H2SO4 in water and dilute to 1000 ml. Stock nitrite solution: Dissolve 1.232 g NaNO2 in water and dilute to 1000 ml; 1.00 ml = 250 µg N. Preserve with 1 ml CHCl3 . Intermediate nitrite solution: Prepare an intermediate nitrite solution having 50 ppm concentration of NO2-N from stock nitrite solution. Standard nitrite solution: Dilute 10.0 ml of the intermediate nitrite solution (50 ppm) to 1000 ml with water; I ml = 0.50 µg N = 0.50 ppm = 50ppb Standard potassium permanganate titrant, 0.01 M (0.05 N): Dissolve 1.6 g KMNO4 in 1 liter distilled water. Keep in a brown glass-stoppered bottle and age for at least one week. Standardize this solution frequently. PROCEDURE: If sample contains suspended solids, filter through a 0.45 µm pore diameter membrane filter. If sample pH is not between 5 and 9, adjust to that range with 1N HCl or NH4OH as required. To 25 ml sample, or to a portion diluted to 25 ml, add 2 ml color reagent and mix. Between 10 min. and 2 hr after adding color reagent, measure absorbance at 543 nm. Preparation of calibration standards from working standard of 50 ppb Standard Solutions Volume (ml) used of the working standard, diluted to 25 ml. Standard Conc. ( ppb NO2-N ) ZERO 5.0 10.0 25.0 BLANK 10 20 50 Calculation: Prepare a standard curve by plotting absorbance of standards against NO2-N concentration. Compute sample concentration directly from curve. DETERMINATION OF NITRATE-N IN WATER APPARATUS: pH meter; Nitrate combination electrode; Stirrer; 120 ml beakers REAGENTS: Stock Nitrate Solution: Dry potassium nitrate (KNO3) in an oven at 1050 C for 24 hours. Dissolve 0.7218 g in water and dilute to 1000 ml. This makes 100 mg/l NO3-N Intermediate Nitrate Solution: Dissolve 10 ml of 1000 ppm NO3-N solution to 100 ml; 1 ml = 100 µg/ml = 100 ppm Buffer Solution Dissolve 17.32 g Al2(SO4)3.18H2O, 3.43 g Ag2SO4, 1.28 g H3BO4, and 2.52 g sulfamic acid in about 800 ml water. Adjust to pH 3.0 by slowly adding 0.01 N NaOH. Dilute to 1000 ml and store in a dark glass bottle. Sodium Hydroxide Solution NaOH 0.1 N Reference Electrode filling solution Dissolve 0.53 g (NH4)2SO4 in water and dilute to 100 ml. PROCEDURE: Preparation of calibration standards from 100 ppm stock NO3-N solution: Standard Solutions Volume (ml) used of the working standard, diluted to 100 ml. Standard Conc. ( ppm NO3-N ) ZERO 0.2 0.5 1.0 5.0 BLANK 0.2 0.5 1.0 5.0 Preparation of calibration curve: Transfer 0.2, 0.5, 1.0, and 5 ml of intermediate NO3N solution to 100 ml beaker and make volume to 100 ml. Add 1 ml buffer solution, add 0.5 g of Ag2SO4 solid and stir. Lower the electrodes and take the mV reading when it is stable. These will give 0.2, 0.5, 1.0, and 5.0 of NO3-N respectively. Plot potential measurements (Rel mV) against NO3-N concentrations on semilogrithmic graph paper, with NO3-N concentrations on the logarithmic axis (abscissa) and potential (in millivolts) on the linear axis (ordinate). A straight line with a slope of +57 ±3 mV/decade at 250C should result. Measurement of samples: Transfer 100 ml sample to a 100 ml beaker, add 1 ml buffer solution and stir. Measure standards and samples at about the same temperature. Immerse electrode tips in sample and record potential reading when stable. Read concentration from calibration curve and multiply with dilution factor if any. *Note. Add 0.5 g of silver sulfate to all samples and standards if the chloride concentration is above 200 ppm.