Sediments and Sedimentary Rocks

Weathering and Erosion:
The Formation of Sediments and Soil
I. Differences between the earth and the moon:
Earth is tectonically active
– diastrophic movement is the continual uplift, folding, and breaking of the earth’s surface.
Subsequently, it is “torn down” by the surface processes of weathering and erosion .
The earth has a strong enough gravitational force to retain an atmosphere and surface water.
The hydrologic cycle drives most of the surface processes of weathering and erosion.
II. Define Weathering and Erosion -
Weathering -
“The decomposition and disintegration of rocks and minerals at the Earth’s surface by mechanical and physical processes. Weathering processes involve very little or no movement
(or removal of) decomposed earth material.
Erosion
– “The removal of weathered rocks and minerals from the place where they formed. Forces or transporting agents involved in moving disintegrated earth materials are:
1. Water
–
running water such as streams, rivers, etc.
2. Wind – prevailing winds, tornadic storms, sea breezes, etc.
3. Gravity
–
The influence of gravity causing landslides, avalanches, etc.
4. Ice
–
in the form of glaciers
III. Modification of the Earth’s Surface -
Weathering, erosion, and transportation of earth materials
Surface processes continually wear away rocks and landforms
In geologic time they combine to wear away entire mountain ranges, reducing them to flat, low-lying plains.
IV. Types of General Weathering -
1. Mechanical (or Physical) Weathering
–
The physical disintegration of rock into smaller and smaller pieces. The chemical composition of the rocks and minerals are not altered .
The particles formed are called clastics
(meaning “broken”)
2. Chemical Weathering
– occurs when air and water react chemically with rocks to alter their composition and mineral content. The final products not only differ physically from the parent material, but they are different chemical substances. (i.e.
Limestone dissolving by acid rain releasing its calcite content as ions.)
3. Differential weathering - Rocks weather by both mechanical and chemical processes occurring together. Since rocks are not homogenous in composition, usually parts weather at different rates. This is called differential weathering resulting in an uneven surface.
4. Spheriodal weathering
–
Because of differential weathering, the surface of rocks is many times sharp and angular, or cuboidal.
These corners formed on the rocks are “attacked” from all three sides resulting in a “ rounding
” of the angular piece. This is spheriodal weathering .
V. Types of Mechanical (Physical) Weathering -
1. Frost wedging
–
Water expands upon freezing. If water seeps into cracks in the rock and freezes, the ice formed exerts pressure along the crack, expanding the crack, or breaking off a piece of rock. Many times the broken piece remains in place until the spring thaw, resulting in areas (such as mountain passes) of rock fall hazard . The loose angular rock debris at the base of mountains and cliffs is termed tallus .
2. Salt Cracking
–
Whenever salt water evaporates, the salts reform crystals. If water containing dissolved salts enters a crack in the rock and then evaporates, the pressure created by the
newly forming salt crystals can break the rock. This is salt cracking and is common in deserts and shorelines. This is why it is not a good idea to salt driveways or sidewalks to rid them of ice. The concrete will eventually break apart.
3. Abrasion – This is the mechanical wearing and grinding on rock surfaces by friction and impact with other rock materials. This gives the rocks a rounded appearance. This occurs in flowing water, wind actions (i.e. natural sand blasting), and glaciers.
4. Organic Activity
–
Plant roots can crack rock material by the hydraulic pressures associated with root growth. Also, burrowing animals can contribute to rock disintegration.
5. Pressure Release Fracturing
–
Rocks buried deep within the earth are under the pressure of the overburden (country rock). As the overburden is eroded away, the internal pressures of a granite pluton cause it to expand. This causes the surface of the granite to split and crack forming sheets and blocks of rock at the surface in a process known as exfoliation . This may also occur in rocks that are porous such as feldspar rich granites. Water may be
“absorbed” by the feldspars causing them to swell and crack.
This process of swelling by the addition of water is called hydration . This is one of the processes that can turn feldspars into kaolinite , a major clay-forming mineral.
6. Thermal Expansion and Contraction - Heat causes matter to expand and cold causes matter to contract. Surface rocks exposed to the intense heat of the daytime sun heat up and expand. At night when it is cooler, the rocks contract. This constant expansion and contraction over many years causes the rocks to break apart. Enchanted Rock in central Texas was named so because of the cracking sounds it is supposed to make during this process.
VI. Types of Chemical Weathering -
1. Oxidation
– reactions with oxygen
– rusting:
4 Fe + 3 O
2
2 Fe
2
O
3 iron + oxygen = iron oxide
Oxidization reactions are common in nature and usually turns useful material into wastes. This is most common in iron bearing mafic minerals such as olivine, amphibole, and biotite.
2. Corrosion – reactions involving oxygen, water, and CO
2
found in the air and water. Combinations of these can cause corrosive chemical conditions that can chemically weather rocks.
3. Weathering by Solution – dissolution whereby ions disperse into water. I.e. rivers flowing across limestone can dissolve Ca + and CO
3
and carry these ions away.
4. Acids and Bases – Acids are solutions with an abundance of free hydrogen ions (H + ), while bases are solutions that have an abundance of free hydroxyl ions (OH ). Acids and bases dissolve minerals by pulling atoms out of crystals. Carbonic Acid
(H
2
CO
3
) is formed in abundance in nature whenever CO
2 dissolves in some rivers and streams.
5. Acid Rain
–
During storms, Nitric Acid , (H
2
NO
3
) is formed by lightning breaking apart N
2
in the atmosphere into N + N. This combines with water to form nitric acid causing rainwater to naturally become slightly acidic with a pH of 5.5 – 6.5.
Pollutants in the atmosphere such as sulfur dioxide gasses can also contribute to acid rain.
Soil: One Product of Mechanical and Chemical Weathering
I. The Components of Soil -
1. Regolith - the loose, unconsolidated, weathered rock overlying the bedrock. Since different geographic locations have their own unique geologic histories with different rock chemistries, there are different bedrocks and different regoliths resulting in a broad variety of soil types worldwide.
2. Soil – (Pedal = Greek for “soil”); Earth material that has been so modified and acted upon by chemical, physical, and biologic agents that it will support rooted plants.
3. Soil terms
–
Loam
– a mixture of sand, silt, and clay sized particles, along with organic matter.
Litter
– plant or animal matter before decay processes.
Humus
– term for when litter decomposes sufficiently that it can no longer be identified.
4. Soil Profiles
–
Horizon
– the uppermost layer of a mature soil that is composed largely of litter and humus with relatively small amounts of minerals.
A Horizon – is a mixture of humus and minerals in the form of sa nd, silt, and clay. Layers “O” and “A” horizons are referred to as topsoil .
B Horizon
–
is a transitional zone between the topsoil and the weathered bedrock below. Roots and other organic matter may be present but generally the organic content is low.
C Horizon
– this lies directly on unweathered “parent” bedrock and consists of partially weathered rock.
5. Dissolved Material
–
Leeching
– the downward movement of dissolved minerals by downward moving water (i.e. rainwater)
Zone of Leeching
– the “A” horizon is called the zone of leeching where clay and dissolved ions are removed.
Zone of Accumulation - the “B” horizon is called the zone of accumulation where clay, dissolved ions, and water accumulates.
6. Soil-Forming Factors -
Parent Rock – the nature of the soil is partially dependent on the nature of its parent rock, including the texture of the soil and its nutrients.
Time – It has been estimated that for the creation of 1 inch of topsoil, natural processes need around 100 years.
Climate – the upward migration of water by evaporation, root absorption, and capillary action are all factors determining the soil type in areas that have different climates. Soils worldwide are categorized into three main soil types as to the three main climates condusive to soil formation. A very many distinct soil types exist in the world.
1. Pedocals
– desert soils where there is an accumulation of dissolved minerals, calcium, magnesium, and sodium. Deserts typically receive
10 inches of rainfall per year, and many times all at once over a couple of days. This carries dissolved minerals downward forming caliche or hard pan layers comprised of calcium carbonate. This also causes salinization of the soil (an accumulation of salts) which limits that amounts of vegetation that can grow. This in turn reduces the ability to form a good “O” horizon.
2. Pedalfers – humid soils of a more temperate climate. There is a complete loss of the more soluble ions of calcium, potassium, magnesium, and sodium. Less soluble ions are left such as aluminum and iron.
3. Laterites
–
tropical soils formed in areas of great amounts of rainfall. All of the silicon and soluble ions are removed leaving only aluminum, oxygen, and
water. The mineraloid bauxite (a major aluminum ore) forms here.
7. Rates of Growth and Decay of Organic Matter - This is related to the accumulation of humus:
Temperate latitudes are ecologically balanced that thick layers of humus occurs resulting in the most fertile soils .
Tropical latitudes have so much water that decomposition by bacteria, mold and other fungi that decomposition is so rapid that very little humus level forms.
Deserts have so much salinization that abundant plant life cannot be supported so very little or no humus develops.
Polar regions are condusive to such slow plant growth that little humus forms.
8. Slope Angle and Aspect - Valley floors have the deepest and richest soils due to the fact that soils tend to “creep” down slope.
Exposure of a slope to the sun also affects soil formation.
9. Soil Erosion and Agricultural Systems
–
Rates of erosion are dependent upon vegetation, litter, humus, and amounts of rainfall. Erosion increases by the removal of the ground cover (usually vegetation).
Deforestation results in the loss of topsoil due to runoff.
Today, the erosion rates exceed the rate of topsoil production by about 35% in the world’s croplands.
Silt runoff into major river systems causes near continent oceanic waters to become turgid, reducing the amount of photosynthesis by phytoplankton.
Sediments and Sedimentary Rocks
Most fossils are found in sedimentary rocks. This is because the organic remains of organisms are usually destroyed by the high temperatures associated with igneous activity or the processes of metamorphism. The type of sedimentary rock formed in an area reflects the environment in which it was deposited . The term used by geologist to describe this aspect of sedimentary beds is “ facies
”. Much can be learned about the ancient environments of the earth by studying various characteristics of sedimentary rocks.
All rocks form initially with the solidification of molten magma or lava .
These newly formed igneous rocks are subsequently subjected to the surface processes of weathering and erosion (the destructive actions of running water, wind, glaciers, etc.) These rock fragments eventually settle out somewhere to form “ sediments ”. These sediments can become compacted to form sedimentary rocks . If these “new” sedimentary rocks are subjected to enough heat and pressure, they may become changed into “ metamorphic ” rocks. If the sedimentary rocks are completely melted by geologic processes, they revert back into a type of igneous rock upon cooling.
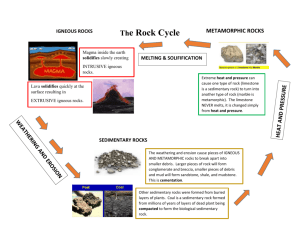
I. The Rock Cycle:
The rock cycle is the conversion of one rock type into another by melting, pressure deformation, and weathering and erosion. All rocks are initially igneous (The word “
Igneous
” means “ born of fire
”). Surface processes can then weather and erode these igneous rocks into sediments that can form sedimentary rocks. Both igneous and sedimentary rocks being subjected to intense heat and pressure can form metamorphic rocks. All three rock types after being subjected to intense temperature can reform igneous rocks.
II. Rock Types:
Igneous rocks make up 90% by volume of the earth's crust. Igneous rocks are formed directly from molten material having its origin in the interior of the earth. As this molten material cools in some areas, it solidifies and hardens to become rock. Intrusive igneous rock forms below the surface of the earth. Extrusive igneous rocks form from molten material that has been forced out onto the surface of the earth (i.e. volcanoes).
Sedimentary rocks form from the accumulation of eroded debris of other rocks or chemically from elements in seawater. Sedimentary rocks make up 75% of all of the rocks exposed at the earth's surface and are where most all fossilized remains are found. This makes sedimentary rocks useful in interpreting the earth's geologic history.
Metamorphic rocks are formed from pre-existing rocks that have been altered as the result of intense heat and pressure. Metamorphism increases the “ crystallinity “ and hardness of the rock; sandstone changes to quartzite; shale changes to slate, and limestone changes to marble.
III. Types of Sedimentary Rocks:
Since the facies of sedimentary beds tells the geologists so much information about the geologic past (paleoenvironments, paleoclimates, and past life forms), sedimentary rocks are emphasized in Historical
Geology. There are 2 basic groups of sedimentary rocks :
1. Chemical Precipitates from the evaporation of seawater, or from the concentration of ions in water. These include rocks such as limestone and various salts such as Halite (NaCl), Sylvite (KCl), Gypsum
(CaSO
4
), etc. The salts usually indicate periods of massive evaporation of aqueous environments.
2. Clastic Sedimentary Rocks are formed from the accumulation of debris from the weathering and erosion of other rocks. The 4 stages of the formation of clastic sedimentary rocks (“ clastic
” means " broken ") are described on the following pages.
IV. The Four Steps for Formation of Sedimentary
Rocks:
1. Physical and Chemical Weathering of the “ Parent Rock ” (the source rock from which the clastic material is being derived). Physical weathering includes the breaking apart of the parent rock by freezing and thawing, wind erosion, etc. Chemical weathering includes dissolution of the parent rock by chemicals in the water (i.e. acid rain).
2. Transportation is the stage where the clastics are
" moved "(“ transported ”) from the source area by water, wind, gravity, or ice . The terrain determines the area of transportation. The distance the particles are moved depends on the amount of energy operating in the environment. It would take more energy to move a boulder than a grain of sand. The larger the sediment size, the more energy is needed to move it. High- energy environments would include white water mountain streams that are capable of moving almost all sizes of particles. Low-energy environments include lagoons, lakes, deltas, swamps, etc., that are capable of moving only the smaller particles.
3. Deposition is the stage where the sediment is deposited in a particular geographic environment, which constitutes the sedimentary environment . As in transportation, the area of deposition is also determined by terrain. For example, large rocks formed on a mountain range would be carried down the steep gradient and deposited at the
base of the mountain if the energy of the stream carrying them decreased when it reached the base of the mountain. Since the stream no longer has the high energy from the gradient, the large rocks are deposited in a manner indicative of a mountain stream environment.
Sedimentary rocks can be interpreted to find out the environment in which they formed.
Sedimentary Environments can be divided into several categories:
Shoreline and Coastal Environments
“ Fluvial ” or Stream, River, and Delta Environments
Alluvial Fans or deposits at the bases of mountains
“Aeolian ” or “wind-borne” deposits
There are numerous other sedimentary environments that your instructor will inform you of at the appropriate time
4. Compaction is the final stage in the formation of a sedimentary rock. At this stage the sediments are compacted due to the weight of the overburden (overlying sediments) and can be eventually “ lithified ”
(turned to stone) as the particles are cemented together with substances such as Calcite (CaCO
3
), Silica (SiO
2
), or forms of Iron Oxide (i.e.
Fe
2
O
3
), among other compounds..
V. Properties of Clastic Sediments:
These include certain characteristics of the sedimentary rock that give specific information about the environment of deposition. These include particle size, degree of roundness, degree of sorting, and color.
1. Particle Size: Clastic sediments are found in various sizes ranging from <1/256 mm to >256 mm. Refer to Figure 1. The Wentworth
Scale of Particle Sizes. The name of a particular sediment size is based on its particle size rather than its chemical composition. For example, "sand" refers to particles having a size range between
0.125mm – 0.5mm. There can be quartz sand such as that found along the Gulf Coast or there may be feldspar sands, gypsum sands, etc.
Remember that sediment size indicates the amount of energy operating in the depositional environment and is therefore a useful clue in determining what the sedimentary environment was. Boulders represent a high- energy environment such as a river channel while clays represent a low energy environment such as a floodplain or swamp.
The Wentworth Scale of Particle Sizes that is a list of sediment particle sizes and the names used to describe them:
The Wentworth Scale of Particle Sizes
Particle Name Approximate Particle Diameter in millimeters
Boulders
Cobbles
Pebbles
Granules
Very Coarse Sand
Course Sand
Medium Sand
Fine Sand
Very Fine Sand
Silt
Clay greater than 256mm
128
64
32
16
8
4
2
1.0
0.5
0.25
0.125
0.0625
0.0313
0.0156
0.0078
0.0039
Fractional Equivalents
1/2
1/4
1/8
1/16
1/32
1/64
1/128
1/256 less than 1/256
2. Roundness: This is simply how “round” (or smooth) the particles in the rock are. Particles in rocks that are angular, irregular in shape, and have sharp edges are called “ poorly rounded ”. Particles that are smooth and have no edges are called “ well rounded ”. The degree of roundness indicates either the amount of agitation the particles were subjected to before deposition, or the length of time it took to transport the particle. “Well rounded” particles indicate that the particles were subjected to a high amount of saltation (bouncing along as they were transported) or being transported for a very long distance such as from
the center of a continent to its shoreline. Both of these factors indicate how much the rock particle was hit by other fragments or was saltated along the route of transportation. “Poorly rounded” sediments indicate either a low amount of agitation, or a short distance of transportation from the time the particle weathered or broke away from their parent rocks . A high-energy environment, which allows for a long period of exposure to weathering, such as a beach or in a stream, is condusive to the formation to the formation of “well-rounded” sediments. On the other hand, a high-energy depositional environment that does not allow a long period of exposure to agitation, such as an alluvial fan, prevents the sediments from becoming “well-rounded”
3. Sorting: refers to rock fragments separated according to particle size. “ poorly sorted ” sediment would contain particles of varying size.
This usually represents a rapid deposition as the result of a rapid decrease in the energy of an environment. Poorly sorted sediments are many times found in alluvial fans at the base of a mountain. This results in a " dumping effect " of sediments at the base of the mountain
( high- energy to low- energy ). “ Well Sorted ” sediment contains material that is made up primarily of all the same sized particles. This indicates that the rate of deposition is slow enough to allow the materials to be separated. Of course, the energy of the environment must be sufficient to accomplish this. Beaches, such as those along the Texas coast, allow sorting to occur. The high energy from the waves combined with a proper depositional rate provides excellent conditions for sorting of the sediments. Sediment is said to be " Mature " if it is well rounded and well sorted. Poorly sorted and poorly rounded sediment is said to be " Immature ".
4. Color: The color of sediment can provide useful information about a sedimentary environment. In general, colors of sedimentary rocks can be interpreted in the following manner: a.) Red, yellow, brown - oxidation conditions, probably marine in origin. b.) Black, gray, greenish-gray - reducing conditions, probably marine except for floodplains and swamps.
c.) Light gray or white - little iron present, either marine or nonmarine; other characteristics of the rock must be considered such as the presence of fossils, the type of fossils, whether or not there is cross-bedding, etc.
VI. Chemical Precipitates:
Chemically formed sediments are produced under various conditions, but generally speaking, when seawater becomes saturated with chemicals, they will precipitate out of solution. This is similar to when a lot of sugar is added to hot tea and then it is allowed to cool. Some of the sugar will " crystallize " or settle out of solution because the tea was
"saturated" with sugar and it could not stay dissolved. Precipitates usually form only in low energy environments such as lagoons or deep-sea environments. Chemical Precipitates would not be found in high- energy environments.
Limestone and Dolostone
– These “carbonate rocks result from the concentration and precipitation of Ca + , Mg + , and CO
3
ions in the sea.
Limestone - Ca CO
3
(primarily calcite) forms offshore from the precipitation of calcium and carbonate ions that have been dissolved off of the continents. Limestones may also be formed from the accumulation of microscopic calcareous tests (shells) of planktonic (or other aquatic level) micro-organisms.
Dolostone - Ca,Mg (CO
3
)
2
(primarily dolomite) forms in a similar manner, but contains magnesium as well as calcium. Dolostone may start off as limestone and later is subjected to groundwater replacing Ca + with Mg + . Or, some dolostones indicate having formed the calcium/magnesium carbonate all at once.
“
Bioclastic sediments
” are formed by living organisms. Many aquatic marine organisms produce shells or other protective coverings by secreting calcium carbonate (limestone) or calcium magnesium carbonate (dolomite) . When these organisms die, their shells accumulate along the sea floor forming layers of broken shell fragments.
Such material is biochemically produced and is ultimately broken by water action They are then referred to as " bioclastic sediments ". The sedimentary rock coquina is a good example of a bioclastic deposit.
The availability of nutrients decreases the further from the shore therefore most marine organisms live in the coastal, shallow water areas. As the distance from shore increases, generally the number of marine organisms decreases. The facies of bioclastic sediments such as coquina usually indicates a beachfront .
“Organic Rocks” form as the result of organics (such as vegetative matter) accumulating in low energy, reducing, anaerobic environments such as swamps. The material does not rot quickly and the volatiles are driven off leaving behind the carbon. A good example of an organic rock is coal . The first stage is called peat . As the peat gets compressed over time, it becomes lignite coal . As lignite becomes compressed, it becomes bituminous coal . As bituminous coal becomes compressed, it forms the metamorphic rock anthracite , the final stage of coal. Other types of organic rocks may form from accumulations of dead organisms (such as fish) in low energy lagoons.
VII. Bedding or Layering of Sedimentary Materials:
Sedimentary rocks are deposited in layers known as " beds ". The type of bedding will vary depending on the environment of deposition. Under normal conditions, beds are deposited in horizontal layers with the bedding planes (the line of contact between the beds) parallel to one another. " Cross-bedding " occurs when the surface of deposition is inclined (i.e. a delta) or a current is present (i.e. a stream). This type of bedding is called " cross-bedding " and is indicative of these environments.
The types of currents that form cross-bedding strata are: a. Aeolian - wind action b. Fluvial - river and stream action c. Marine in Origin - current action
Types of cross-bedding include planar - the bedding planes separating the cross-bedded units are parallel, wedged - the bedding planes are at an angle to one another and form a wedge; and trough - the bedding planes separating the cross-bedded units are curved.
Thick planar or wedged cross-bedding always indicates an aeolian
(wind) deposit such as a sand dune in the desert. Thin planar or wedged units may be aeolian , fluvial , or marine . Because of this, other characteristics such as color must be used to determine the environment of deposition.
Many times paleocurrents of water (and sometimes wind) can be traced by the ripple marks left in some sedimentary rocks indicating ancient river channels or beachfronts. Mud cracks can also be preserved indicating ancient low energy mud flats.
Another type of bedding is known as graded bedding . This is where there is a gradation in the size of particles within a unit of deposition.
Larger particles are found on bottom with successively smaller sediments on top. This type of bedding is formed by " turbidity currents ", which are the sudden flows of material down the continental slopes. This causes the finer particles to be suspended in the water while the larger particles fall out and are deposited on the bottom with smaller and finer sediment on top. This results in a " gradation " in particle size. The facies of graded bedding is deep water marine.
VIII. The Marine Lithofacies:
This refers to the depositional sequence found in a cross section of a shore to deep- water environment. The usual sequences of rock types are:
1. Sandstone formed on beach areas
2. Siltstone formed near-shore
3. Claystone/Shale formed further out
4. Limestone formed even further out in deeper waters
A schematic of the typical marine lithofacies is as follows:
The Marine Lithofacies
Transgression: - the advancement of the sea onto the land because of a worldwide increase in sea level or a subsidence of the landmass.
Regression: - the retreat of the sea from the land due to a worldwide drop in sea level or the uplift of the land.
Transgressional and Regressional sequences of strata can be used to interpret and retrace ancient coastlines.
Transgressional Sequence Regressional Sequence
Metamorphism
Metamorphism – From the Greek “meta” = to change, and
“morpho” = shape.
Metamorphism – “The altering of rock characteristics and mineral compositions due to heat and/or pressure, or other environmental factors. This changing is a Solid State Reaction , meaning that the rocks subjected to metamorphic processes do not melt (otherwise upon cooling, they would form igneous rocks). It is thought to be a relatively slow geologic process. A great many areas of metamorphism yield abundant mineral reserves of gold, silver, copper, lead, zinc, and other valuable minerals.
Metamorphic rocks are formed either by being exposed to heat, pressure, or chemically active fluids , or a combination of these factors to create a rock that has a different texture and mineral content.
The “ parent rock ” is the term for the rock prior to metamorphism. It may be igneous, sedimentary, or another metamorphic rock. For example, here are some parent rocks and the rock that they may metamorphose into under certain conditions:
Limestone
– marble
Clay stone
– slate
Granite – gneiss, etc.
The effect of metamorphism on rocks is analogous to baking a cake: the resulting cake is dependent upon the ingredients, the amount of fluids, the temperature, and the length of time it was “baked”.
A great portion of the continents is metamorphic formed during
“ continental accretion ” during the formation of the Precambrian.
Metamorphics form the stable basement rocks called
“continental shields”
upon which surface sedimentary rocks have been deposited.
Metamorphics also comprise a large portion of the crystalline core of many mountain ranges.
Factors Involved in Metamorphism
I.
Heat
– The source of heat may be from a large intrusive body such as a pluton, or heat from activities associated with s plate tectonics.
At temperatures below 200 0 C, only a small amount of fluid is present in most rocks. As the temperature increases many minerals release pore fluid that was trapped in the rock or in crystal lattices of its minerals. This pore fluid may become very chemically reactive, altering the chemistry of the surrounding rocks.
The Geothermal Gradient
– On average the temperature of the rocks in the earth increase 25 0 C per kilometer of depth . On the continental cratons, the average is 20 0 C/km. On the continental boundaries it is 40 0 C/km. At subduction zones, it is 10 0 C/km because heat is dissipated into the sea.
At 700 0 C, most rock components become “ plastic ” where many times the pre-existing crystals rotate , or twist altering the texture of the rock.
Under conditions of high heat, pressure, and chemically active fluids, crystal lattices begin to break down, recreate new types of crystal lattices, rearrange ions, and form new minerals in the process.
Some minerals only form at certain temperature and pressures.
If these are found in a metamorphic rock, the temperature of formation can be deduced.
II.
Pressure
– When rocks are buried, they are subjected to lithostatic pressure that is the pressures from all sides by the overburden weight of the country rock...(This is similar to the intense pressure increases experienced by going deeper and deeper in water).
Differential pressures
– may exist whereby the pressures exerted upon the rock are not equal in all directions. This results in a distortion or twisting effect on the rock.
Phenocryst rotation or distortion may occur. This can cause grains in the rock to stretch, rotate, bend, line up in rows, become platy, etc. (i.e. micas forming in mica schists)
Pressure distortion of metamorphic rocks is common around areas of high lithologic stress such as areas around tectonic boundaries.
III.
Chemically Active Fluids –
Fluids released from igneous intrusions, or other metamorphic processes can cause a constant interaction or exchange of ions altering the rocks. i.e. 2Mg
2
SiO
4
+ 2H
2
O
Mg
3
Si
2
O
5
+ MgO
Olivine Water Serpentine carried away in solution
Metasomatism
– the introduction by fluids of ions from an external source not directly associated with the intrusion.
Hydrothermal Metamorphism
– changes due to migrating superheated water and dissolved ions. Hydrothermal rocks many times appear “bleached” because of the intense chemical reactions.
Sources of water -
1. Juvenile Water
– water given off by cooling magma.
2. Metamorphic Water – water already present the country rock, which is given off during metamorphic processes.
3. Meteoric Water – “groundwater” contained in aquifers encountered in the country rock during metamorphic processes.
Hydrothermal activities – many times form economically rich mineral deposits of gold, copper, iron, lead, etc. This process is also responsible for the “veining” (“mother loads”) of gold and other valuable minerals.
Volcanic activities such as calderas usually have associated hydrothermal activities resulting in mineral enrichment.
The Three Sources for Chemically Active Fluids in
Metamorphism:
1. Water trapped in the pore spaces of sedimentary rocks as they form
2. Water arising as volatile fluid within magma
3. Water from the dehydration of water-bearing minerals such as Selenite Gypsum: CaSO
4
2H
2
O, and some clays.
Types of Metamorphism
I. Contact – Effects of Heat and Fluids
Characteristics:
“Heat” is the driving force in contact metamorphism.
Common where hot magmatic plutons come into contact with the surrounding country rock.
The degree of metamorphism is related to the temperature of the magma, the size of the intrusion, and the chemically active fluid content of the magma involved. Large intrusions such as batholiths cool for long periods of time so there is usually a more intense metamorphic change in the country rock.
Temperatures can reach 900 0 C next to the intrusion.
As the heat and associated metamorphic changes alter the country rock, the country rock closest to the intrusion is affected most , and the furthest from the intrusion is affected least .
This sets up a “ metamorphic halo ” or “ aureole ” in the country rock around the intrusion.
The aureole is a gradation of degrees of metamorphism surrounding the intrusion such as the following:
1. Shale – unaltered country rock
2. Slate
– low grade metamorphism
3. Phyllite –between low and medium grade
4. Schist – medium grade metamorphism
5. Gneiss
– high grade metamorphism
6. Migmatite – very high grade metamorphism
7. Melting occurs at above this temperature resulting in the formation of an igneous rock .
Two types of contact metamorphic rocks are recognized:
1. those resu lting from the “ baking ” of the country rock
2. those resulting from the actions of chemically active fluids
Many “ baked
” types have the texture of porcelain if they contain high amounts of clay such as shale. This effect is seen in the firing of ceramics in a kiln.
Hydrothermal activity is also common with contact metamorphism resulting in an enrichment of valuable ore deposits. This occurs during the final stages of cooling, whenever the magma begins to crystallize. Large amounts of hot, watery solutions are released. This process usually occurs near the surface of the earth, also resulting in the enrichment of minerals such as gold, silver, copper, lead, etc..
II. Regional Burial – Effects of Lithostatic Pressure
Characteristics:
Occurs over a very broad area
Rocks are altered due to tremendous pressures (and the resulting high temperatures), resulting in deformation within deeper portions of the crust.
Very common along convergent and divergent plate boundaries.
Index minerals are minerals that are known to form only under certain temperatures and pressures. The following is a sequence of known minerals that form from low grade metamorphism to high grade: chlorite – (forms around 200 0 C), muscovite, biotite, garnet, staurolite, kyanite (forms around 500 0 C)
Quartz and feldspars can be present in both igneous and metamorphic rocks, but some minerals such as andalusite, sillimanite, and kyanite (all 3 minerals are forms of Al
2
SiO
5
) form only from these metamorphic conditions.
The presence or absence of these minerals is an indication of the degree of pressure (and resulting heat) in the formation of the rock in question.
Examples of regional burial rocks are: marble from limestone, quartzite from quartz sandstone, and argillite from clay.
III. Dynamic Metamorphism (“Dynamo-thermal”)-
Characteristics:
Usually associated with the pressures around fault zones .
“Mylonites” is the term used to describe rocks formed in this way.
Typically, the extent of metamorphism is restricted to narrow margins adjacent to faults.
Myolinites are hard, dense, fine-grained rocks, many of which have laminations or layerings.
These also can be associated with tectonic settings.
Textures of Metamorphic Rocks
I. Foliated Textures -
Characteristics:
Typically associated with contact metamorphism .
Minerals are arranged in a platy, parallel fashion.
The size and shape of the mineral grains determines if the foliation is fine or coarse .
A coarse foliation usually indicates a higher degree of heat such as in gneiss.
A fine foliation usually indicates a lower degree of heat such as in schist.
Slate is very fine foliation exhibiting the lowest grade of contact metamorphism.
Examples of Foliated Textured Metamorphic Rocks:
1. Slate
–has a very fine foliation due to it having formed at the lowest grade of contact metamorphism. It possesses a slaty cleavage , easily cleaving or parting along the axis of layering. It is used for pool tables, chalkboards, and building tiles for this reason. The different colors of slates are due to the presence of minerals such as chlorite (green), graphite (black), or iron oxide
(red).
2. Phyllite – similar to slate but coarser grained. It is more lustrous or glossy due to tiny mica minerals. Grains are too small to be identified with the unaided eye.
3. Schist
–
is most commonly produced by regional burial metamorphism. It can also be produced by medium grade contact metamorphism. Metamorphosed clay rich sedimentary rocks typically produce schists (although other rocks may also produce them). All schists contain more than 50% platy and elongated minerals all of which large enough to identify. The degree of schistosity reflects the temperature of formation: the greater the temperature, the greater the degree of schistosity.
Schists are common in low to medium grade metamorphic environments. Schists are named as to the most abundant mineral: mica schist, talk schist, biotite schist, chlorite schist, etc.
4. Gneiss
– is a streaked or has segregated bands of alternating light and dark minerals. Quartz and feldspar are the major light colored minerals and biotite and hornblende are the principle dark colored minerals. Gneiss typically forms from regional metamorphism of clay-rich sedimentary rocks, from contact metamorphism of granites, or from metamorphism of older metamorphic rocks.
5. Amphibolite – a dark-colored, slightly foliated rock consisting primarily of hornblende and plagioclase. The metamorphism of mafic rocks such as basalt produce amphibolites.
6. Migmatites
– “mixed metamorphics” – These have characteristics of both igneous and metamorphic rocks indicating very high heat and pressure. Examples include the rocks touching an intrusion: the very highest grade contact metamorphism. Most contain granite components, or lenses (small pieces of other rocks) , and appear to have been twisted or wavy. This may be due to partial melting of the country rock.
II. Nonfoliated Textures -
Characteristics:
These textures result from the metamorphosing of rocks whose minerals do not show a preferred orientation, and therefore are not foliated.
Most non-foliated rocks result from contact or regional burial of rocks that are devoid of platy or elongated crystals.
Two Types of Nonfoliated rocks:
1. those composed of mainly one mineral (marble or quartzite)
2. those composed of mineral grains that are too small to be seen as in hornfels or greenstones.
Examples of Non-foliated Textured Metamorphic Rocks:
1. Marble – the parent rock is a limestone (mostly calcite) or dolostone (mostly dolomite) that was subjected to contact or regional burial. It may be fine-grained to coarse-grained. Color variation is due to impurities in the parent rock. Because of its texture and softness, marble has been used extensively for sculpturing.
2. Quartzite
–
the parent rock is a quartz sandstone subjected to medium to high grade contact or regional burial resulting in a hard, coarse-grained compact rock. Pure quartzite is white but impurities may alter the color. Since it is so hard from the recrystallization of the quartz, it is commonly used for the bases of roads and buildings.
3. Greenstone – this is the name given to any compact, dark green, altered, mafic igneous rock that formed under low to high grade metamorphic conditions. The green color is due to the minerals chlorite, epidote, and hornblende. These are commonly the rocks found in “ greenstone belts
” along the transitional zones of sialic continental plates to mafic oceanic plates.
4. Hornfels
–
fine-grained, nonfoliated rock formed from contact metamorphism. The grains are equidimensional with its composition dependent upon the composition of the parent rock. Most are formed from contact metamorphism of clay-rich sedimentary rocks or impure dolomites.
5. Anthracite
–
is a black, lustrous, hard coal that is high in carbon and low in volatiles. Its parent rock is bituminous coal that was subjected to regional burial.
Metamorphic Zones or Facies –
A “ metamorphic facies
” is a group of metamorphic rocks characterized by particular mineral assemblages (more than one mineral is present) under the same broad temperature/pressure conditions.
Each facies is named after its most characteristic rock or mineral.
Metamorphic facies are usually are applied to areas whose parent rocks were originally clay-rich . Metamorphic facies cannot be applied to areas where the parent was pure limestone or pure quartz sandstones because they would produce only marbles and quartzites respectively.
Examples of Metamorphic Facies:
1. Greenschist Facies
– forms whenever the rock is rich in the mineral chlorite and is subjected to relatively low temperatures and pressures.
2. Granulite Facies and Amphibolite Facies
–
form under similar chemistries but the pressures are significantly greater.
3. Blueschist Facies
–
form at subduction zones where, due to the presence of seawater, the temperature is low , but because of the tectonic activity, the pressure is high . This results in an abundance of a blue-colored amphibole mineral named glaucophane . The presence of a blueschist facies indicates to the geologist the presence of ancient subduction zones.
Geologic Time
I. Geochronology
– the science of dating the earth and events in earth’s history.
There are two main types of dating techniques:
1. Relative Dating
– these techniques determine the order of events…which one happened first, second, third, etc. Relative dating does not tell you how many years ago the event took place.
2. Absolute (Radiometric) Dating – these techniques use the decay rates of radioactive isotopes found in rocks to determine the precise number of years ago the rock in question formed.
II. Founders of Geochronology and Relative Dating
Archbishop Ussher (1600’s) – conscribed by the Pope at the time to figure the age of the earth. He took the Bible and going from Revelations backwards to Genesis, and ascribing a standard life span to the peoples mentioned, he figured that the earth was created on October 26 th , 4004BC, at 9:00 ante meridiem (a.m.)
(post meridiem is for “p.m.”). The last “4” in 4004 is to compensate for a four year mistake whereby it is mentioned in the
Bible that the Magi (Wise Men) traveled to Bethlehem by way of
King Herod’s castle. Herod died 4 BC (“before Christ”) by today’s calendar, making the birth of Christ around 4 BC! So, the second millenniu m AD (Anno Domini = Latin “Year of our Lord”) was in the year 1996, not 2000.
Nicholas Steno – (1600’s) – He was a Danish physician for the
Duke of Tuscany. When not attending the Duke, he hiked around the countryside making notes of his observations of geology: how streams eroded hills, how rocks were deposited, etc. He proposed three ideas that are known as
Steno’s Principles
.
1. Superposition – in any sequence of undisturbed strata, the oldest is on bottom and they are progressively younger to the top.
2. Original Horizontality
–
As rock layers are being
deposited, they are first deposited in a horizontal fashion and then later uplifted, folded, or broken. (He did not take into consideration cross-bedded layers or the near vertical layering at river deltas.)
3. Lateral Continuity – In a sequence of strata, one particular rock layer does not go on forever laterally. There are limiting factors: a. The depositing body, such as a river, may run out of sediments to deposit. b. There may be a geographic barrier (i.e. a mountain range on either side of a river valley) that prevents lateral expansive deposition of a layer. c. The conditions of the energy of deposition may change such as larger parti cles can be carried by the river’s headwaters, but only sand and clay in the river’s path across the coastal plains. This allows for a “feathering out” transition between rock layer types.
James Hutton (1726
– 1797, Scottish Geologist) – “the Father of Geology” – His concept of “ Uniformitarianism ” states that all of the chemical and physical processes that go on today’s earth
(mountain building, volcanoes, erosion, deposition, etc.), also went on in the geologic past. This meant that the earth must be older than the 6000 years accepted by the Church. His Book
Theory of the Earth describes that the earth must be millions of years old, not thousands.
Charles Lyell – a student of Hutton. He is considered to be the
“Father of Geochronology” because of his amendments to uniformitarianism set forth in his book Principles of Geology .
His Principle of Cross-cutting Relationships states that any intrusion or fault that cuts across a body must be younger than the body it cuts. Another principle of his is the Principle of
Inclusions that states any rock included in another rock must be older than the rock in which it is included (i.e. a sandstone may be
10 million years old, but the sand particles, inclusions, must be older because they must have been weathered and eroded from another older parent rock.
Thickness of sediment measurements
–
Both Hutton and Lyell
(as well as others) measured the outcrops of exposed, fossil-
bearing, sedimentary rocks all over Europe. Supposing an average sedimentation rate of 0.3 meters/1000years, and a total thickness of 150,000 meters, they estimated the age of the earth to be around 500 million years. The flaw with this idea is that they were only measuring fossil-bearing strata of the Phanerozoic Eon.
They did not take into consideration transgressive-regressive sequences of the sea, interrupting depositional sequences. Also, because of its sometimes inaccessibility for study, they did not know of the vast amounts of Precambrian strata that represents
88% of earth’s depositional history .
William “Strata” Smith – (1800’s Geologist) –
His concept of
Floral and Faunal Succession states that fossil plants and animals occur in the geologic record in a definite and determinable order, and time periods can be recognized by these fossils. For every geologic time, there is a unique assemblage of plant and animal fossils specific for that timeframe.
Charles Darwin
– the “Father of Evolution” – In his book
The
Origin of the Species , he laid down the concepts of natural selection and evolution of life that in 1859 was accepted and contributed to the acceptance that the earth was considerably older than believed by the Church.
Baron Georges Cuvier
– (1800’s French anatomist and paleontologist) – As an opponent of uniformitarianism, he believed that the Church’s accepted age of the earth was correct.
He believed in Deus Irae (the “wrath of God”). This is the concept of how mountains, valleys, crumpled rock layers, etc. was by God unleashing some catastrophe upon the earth. He came up with the concept of Catastrophism to explain the age of the earth.
John Joly
– (1899) –
He proposed that the earth is 90
– 100 million years old based on salinity measurements of the sea compared to freshwater. He assumed that the seas were originally freshwater. After measuring the (average) salinity of the oceans (35ppt salts), he compared that to the average salinity runoff of rivers. The 90
– 100 million-year estimate is an approximation of the time required to make the sea have 35ppt salts. The major flaw in this reasoning is that he did not consider transgressions and regressions of the sea leaving vast amounts
of landlocked salts as evaporite rocks that would then again be subjected to erosion…sea salts get “recycled”.
Lord Kelvin
–
(1824
– 1907, English Physicist) tried to discredit uniformitarianism by thermal (heat) studies. Assuming that the earth was molten at the beginning, and, knowing the mass and volume of the earth, and that the earth has continued to cool,
Kelvin figured that the earth could not be younger than 20 million years, or older than 400 million years. This broad range of ages for the earth is due to his variability in his temperature data collected in some deep mines in Europe. His major flaw is that he did not take into account the heat created by the decay of radioactive elements that has kept the earth’s interior from cooling. These properties cause the earth not to lose heat at a regular rate.
III. Unconformities
“Geologic time is continuous…deposition of rock layers is not”
The surface processes of weathering and erosion erases depositional evidence.
Deformation of once horizontal beds can create topographic highpoints that are more apt to erode away creating irregular erosional surfaces on the earth .
Later, more deposition can occur on top of these once erosional surfaces.
The irregular line between beds represents the
Unconformity – a hiatus or gap in depositional time .
IV. Types of Unconformities
1. Disconformity – Sedimentary layers are deposited in a horizontal fashion. Then, at a later time, they are exposed to erosion. Subsequently, the erosional surface that was formed gets covered by more sedimentary rock layers.
2. Angular Unconformity
–
Rock layers are deposited in a horizontal fashion, then acted upon by some diastrophic action such as uplift of folding. As these layers erode, and are later covered by more deposition on top, the layers at the bottom remain angular or bent condition while the newer layers on top are horizontal.
3. Nonconformity – This is named so because the rock types do not conform across the erosional surface. If a granite pluton is exposed by the erosion of the overburden or country rock, the granite then begins to also erode. Later if sediment covers the area, there is eroded igneous (or in some cases metamorphic) rock on bottom with sedimentary rock on top.
Relative Dating
is all about utilizing the above principles geologists are able to “interpret” events of a particular outcrop to determine which event came first, second, third, etc. Relative dating is only concerned with the order of events, not the ages of those events.
V. Absolute Dating (Radiometric Dating)
There are 92 naturally occurring elements in nature.
All matter is made up of chemical elements, with each being composed of extremely small particles called atoms .
The nucleus of an atom is comprised of positively charged particles called protons , neutrally charged particles called neutrons , with negatively charged particles, called electrons encircling the nucleus in energy levels or electron shells .
The number of protons in the nucleus of any atom of an element is the atomic number of that particular element. That is the basis of the numbering of the elements on the Periodic Table of Elements.
For instance, the element Hydrogen has one proton in its nucleus.
Therefore it has an atomic number of 1 ; Helium has two protons in its nucleus, and therefore has an atomic number of 2 ; Uranium has 92 protons in its nucleus and therefore has an atomic number of 92 . The atomic number of an element defines that element.
If an element looses a proton by some means, it is no longer that element. Conversely, if an element gains a proton by some means, it is no longer that element.
Neutrons in the nucleus of an atom do not affect its charge (since neutrons are neutrally charged), but neutrons do affect the atomic mass of the element.
The atomic mass of an element is the combined number of protons and neutrons in the nucleus.
Not all atoms of the same element have the same number of neutrons in their nuclei. These variable forms of the same element are called isotopes . For instance, hydrogen has an atomic number of one: one proton and one electron. If a neutron is added to the nucleus of hydrogen, it still has the same atomic number, but you have increased its atomic mass, forming the isotope of hydrogen called deuterium . If another neutron is added to the nucleus it becomes the isotope of Hydrogen called tritium . All three, Hydrogen, Deuterium, and Tritium all have an atomic number of one (one proton in the nucleus), but they are all different isotopes of the same element .
If you could continue to add neutrons to the nucleus of an atom, a point would be reached that the nucleus would become very unstable. Because of our reality being “ruled” by the processes of entropy (whereby everything “wants” to be at its lowest point of equilibrium, or its lowest “rest” state), atoms with unstable nuclei
(those with high neutron to proton ratios) begin to emit particles, which we refer to as radioactivity .
Radioactive decay is the process whereby an unstable atomic nucleus is spontaneously transformed into an atomic nucleus of a different element.
There are three basic types of radioactive decay:
1. Alpha Decay
2. Beta Decay
3. Electron Capture Decay
Alpha decay occurs when 2 protons and two neutrons are emitted from the nucleus, resulting in a loss of 2 atomic numbers and 4 atomic mass numbers.
Beta decay occurs when a neutron in the nucleus emits a fastmoving electron, changing that neutron to a proton and consequently increasing the atomic number by 1, with no resultant atomic mass number change.
Electron capture decay comes about by a proton in a nucleus capturing an electron from an electron shell and thereby converting into a neutron, resulting in the loss of one atomic number, but not changing the atomic mass number.
Some elements undergo only one step to convert from an unstable nucleus to a stable one. Others require several conversions until a stable state is achieved. For example, the element rubidium 87 decays to strontium 87 by a single beta emission, and potassium
40 decays to argon 40 by a single electron capture. Uranium 235 decays to lead 207 by seven alpha steps and six beta steps.
Uranium 238 decays to lead 206 by eight alpha and six beta steps.
The half-life of a radioactive element is the time it takes for onehalf of the atoms of the original unstable parent element to decay into atoms of a new, stable daughter element .
The daughter element is the stable element that an unstable element decays or changes into. The half-life of radioactive elements is constant and can be measured. Each different unstable radioactive element has a different half-life that can range from less than a billionth of a second to 49 billion years.
All igneous rocks contain radioactive isotopes . Whenever they solidify (or cool) the radioactive parent isotope begins to decay into the stable daughter element. So, whenever an igneous rock of unknown age is found, a field sample of it is taken, and the sample is analyzed as to which radioactive isotope is present in abundance. When that is determined, a survey of the daughter element that particular radioactive element decays into is made from the sample in question. This creates a percentage of radioactive parent isotope to the stable daughter isotope present in what is called the parent/daughter ratio for that particular rock.
The half-life (a measurement of time) for that particular radioactive element found in abundance in the field specimen is easily found in physics and chemistry reference books. So, knowing the percentage of the radioactive parent to the stable daughter element present in the sample of igneous rock of unknown age, and knowing the half-life for the radioactive isotope in question, the actual age of the igneous rock can be deduced.
Usually, only igneous rocks can be dated using the following procedures. For metamorphic rocks, only the age of the actual metamorphism can be determined. In rare instances, some sedimentary rocks containing Glauconite (a green-colored, radioactive potassium mineral found in some sedimentary deposits) can give information on the age of deposition of the sedimentary beds. All igneous rocks can be dated using radiometric techniques .
Absolute dating techniques involve the measurement of the breaking down of certain radioactive elemental compounds in the rock that have occurred over time. The rate of decay is known for these radioactive elements from laboratory experimentation.
If a geologist finds an igneous rock layer in the field and needs to know the exact age of the rock, a sample is taken from the outcrop.
This sample is then sent to a laboratory that specializes in radiometric dating techniques. There the rock is ground into a very fine powder. This powder is then analyzed as to which radioactive isotopes are present in the rock. This lab must be equipped with an apparatus called a mass spectrometer . This analytical device allows the geologist to project purified samples of the rock in question into a strong, fluctuating magnetic field that has sensors that can detect the presence of different elements that have different atomic masses. It works similarly to the following scenario. If you turned on a strong fan and stood in front of the fan with a feather in one hand and a lead ball in the other, and simultaneously let go of both, what would happen? The feather would go shooting off because of its low weight (low mass) and the lead ball would fall to the ground because of its high weight (high mass). It’s the same principle whenever the atoms of different masses are projected through the magnetic field of the mass spectrometer: the “lighter” elements “fall” through the magnetic field
differently than the “heavier” elements, there fore hitting the sensors at different areas and different rates. This is how the parent daughter ratio is determined in an unknown sample.
To fully understand this technique, one must be familiar the following terms:
1. Isotope - Varieties of the same element that have different mass numbers. Their nuclei contain the same number of protons but different numbers of neutrons.
2. Parent Isotope - the full amount of isotope in the newly formed igneous rock.
3. Daughter Isotope - (what the parent isotope will eventually turn into) the amount of altered parent isotope over time. The last daughter isotope is stable.
4. Half-life - The time it takes for one-half of the atoms of a radioactive substance (Parent Material) to decay into another element (Daughter Material). For example, Uranium 238
(Parent) decays to Lead 206 (Daughter). The rate of decay is known for many of the naturally occurring radioactive elements. So, if the rate of decay is known, and the ratio of parent material to daughter material is measured in a rock, then the age of the formation of the rock can be found.
5. Mass Spectrometer
– the laboratory device used in determining the relative amounts of residual radioactive
Parent and stable Daughter isotopes.
VI. Other Radiometric Dating Techniques
Carbon 14 Dating –
There are three common isotopes of carbon: 12 C, 13 C, and
14 C.
In the upper atmosphere, nitrogen gas is bombarded by cosmic radiation transforming it into radioactive 14 C. Carbon dioxide, 14 CO
2
, forms. Along with this is 12 CO
2
and 13 CO
2 from other sources such as volcanic eruptions.
During photosynthesis in plants, CO
2
is taken in, and along with water, sunlight, and some pigment such as chlorophyll, sugars are made. sunlight
6CO
2
+ 6H
2
O C
6
H
12
O
6
+ 6O
2 pigment
Of the sugars made, isotopes of carbon are in a ratio of 1/3
12 C, 1/3 13 C, and 1/3 14 C. Other forms of life dependent on sugars produced by photosynthesis for food. As the sugars are eaten and digested, they become incorporated in to the carbon containing compounds of their bodies. As long as they live, the ratio of the carbon isotopes is 1:1:1. Whenever organisms die, the 14 C begins to decay back into nitrogen at a half-life of 5730 years.
Any organic remains may be dated using this method back to around 75,000 years ago making 14 C dating especially useful for archeology.
Tree-ring Dating
–
As trees grow they create rings of xylem tissues representing each year of growth. By counting the rings, the
age of the tree can be determined. By cross-referencing growth patterns from different trees, a timeline backwards can be established. This is particularly accurate back to around 14,500 years ago, again greatly benefiting archeologists.
Fission Track Dating –
As radioactive elements in rocks decay, particles are emitted that leave tiny, microscopic tracks in the crystals of minerals. The older the rock, the more the tracks the crystals contain. By counting the tracks, the ages of rocks formed between 40,000 years ago to 1.5 million years ago can be determined. This method is useful because this time frame is difficult to date: it is too old for 14 C techniques, and many times too young for other radioactive isotope techniques.
Examples of Radiometric Problems
1.) In the geologic past, a rock formed from cooling magma, containing 1 gram of radioactive Uranium 238 and no Lead 206. Many years later a geologist who wants to find the exact age of this rock collects a sample.
If the half-life for U 238 is 4.5 billion years and after analysis the Uranium
238 to Lead 206 ratio (parent/daughter ratio) was 1:1 (50% U & 50%
Pb), how old is the rock?
2.) A rock specimen was found that had a ratio of Potassium-40 to
Argon-40, which was 1:7 (1 part Potassium-40 to 7 parts of Argon-40).
Potassium-40 has a half-life of 1.3 billion years. How old is the rock?
3.) A geologist collects a piece of a meteorite rock in the field and wants to know the exact age of the rock. After close examination of the specimen, it was discovered that the specimen contains sufficient amounts of the potassium 40 to warrant using the K 40 – Ar 40 test.
Knowing that the half-life of K 40 is 1.3 billion years and that there was a ratio of 1 part K 40 to 3 parts Ar 40 , how old is the rock?
4.) If a rock contained a parent/daughter ratio of “parent element X” to
“daughter element Y” of 3:1, and the known age of the rock is 500 million years, what is the halflife of “element X”
VII. The Geologic Time Scale
This is a calendar of sorts stretching from the birth of the earth,
4.6BYA until today. It has taken the work of thousands of scientists and it is still being updated every three years or so as dating techniques become more and more precise.
Study the handout of the geologic time scale focusing on the points mentioned in lecture class.