91392 Sample Assessment Schedule
advertisement

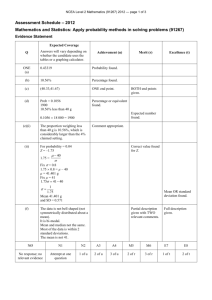
NCEA Level 3 Chemistry 91392 (3.6) — page 1 of 6 SAMPLE ASSESSMENT SCHEDULE Chemistry 91392 (3.6): Demonstrate understanding of equilibrium principles in aqueous systems Assessment Criteria Achievement Achievement with Merit Demonstrate understanding typically involves describing, identifying, and giving an account of aqueous systems using equilibrium principles. This requires the use of chemistry vocabulary, symbols and conventions and may include related calculations. Demonstrate in-depth understanding typically involves using equilibrium principles to explain properties of aqueous systems. This requires explanations that use chemistry vocabulary, symbols and conventions and may include related calculations. Achievement with Excellence Demonstrate comprehensive understanding typically involves elaborating, justifying, relating, evaluating, comparing and contrasting, or analysing properties of aqueous systems in terms of equilibrium principles. This requires the consistent use of chemistry vocabulary, symbols, and conventions and may include related calculations. Evidence Statement One Expected coverage Achievement In (a) (i), equation correct (a) (i) HCOOH + H2O « HCOO– + H3O+ OR in (a) (ii), THREE species correct. Merit In (a) (ii), reasons for species concentrations given. HCOOH > H3O+ HCOO– > OH– (a) (ii) (a) (iii) Methanoic acid is a weak acid so the equilibrium favours HCOOH. Dissociation produces similar amounts of H3O+ and HCOO–. Dissociation of HCOO– produces a small amount of OH–/ water dissociates to form a small amount of H3O+ and OH- in equal amounts and after the dissociation of HCOOH there will be slightly more H3O+ than HCOO-. HCOOH + H2O « HCOO– + H3O+ In (a) (iii), correct calculation of [H3O+]. In (a) (iii), correct calculation. Excellence NCEA Level 3 Chemistry 91392 (3.6) — page 2 of 6 éë HCOO – ùû éë H 3O+ ùû Ka = [ HCOOH ] (10 ) –2.78 2 10 –3.74 = [ HCOOH ] [HCOOH] = 0.0151 mol L–1 (b) HCl is a strong acid and completely dissociates into its ions. .It has a low pH due to a high [H3O+].. In (b), pH is due to H3O+ (or OH–) in solution NH4Cl is an acidic salt that completely dissociates into its ions producing NH4+. NH4+ is a weak acid so partially dissociates into H3O+, however, although acidic its pH is not as low as HCl. The concentration of [H3O+] is lower. OR in (b), conductivity is due to ions in solution. NH3 is a weak base, its reaction with water produces only a limited amount of OH– compared to NaOH which is a strong base and fully dissociates to produce the highest concentration of OH– and hence the highest pH. In (b), difference in pH of two substances explained in terms of H3O+ (or OH–) OR in (b), difference in conductivity of two substances explained in terms of ions in solution. Conductivity relates to the number of ions in solution. Since HCl, NH4Cl and NaOH completely dissociate a large number of ions are produced, hence high conductivity. However since NH 3 partially dissociates there are fewer ions in solution hence low conductivity. Not Achieved NØ No response; no relevant evidence. N1 Candidate provides some accurate statements without answering any question completely. N2 Candidate provides any ONE statement for Achievement. A3 Candidate provides any TWO statements for Achievement. A4 Candidate provides any THREE statements for Achievement. Achievement In (b), properties of THREE substances justified in terms of pH related to H3O+ (or OH–) AND conductivity related to ions in solution of three substances. NCEA Level 3 Chemistry 91392 (3.6) — page 3 of 6 M5 Candidate provides any ONE statement for Merit. M6 Candidate provides any TWO statements for Merit. E7 Either pH or conductivity justified from the Excellence criteria. E8 Candidate provides ALL the evidence from the Excellence criteria. Merit Excellence Two Expected coverage FeS (a) (ii) Ks = [Fe2+][S2-] Let solubility = s (a) (iii) Ks = s2 4.9 ´ Merit In (a) (i) AND (ii), BOTH correct. « Fe2+ + S2- (a) (i) Achievement In (a) (iii), correct answer. 10-18 = s2 s = 4.9 ´ 10 –18 = 2.21 ´ 10-9 mol L–1 [H3O+]2[S2–] = 1.1 [H3O+] = [H3O+] (b) (i) 10-4.20 = 6.31 éëS2– ùû = = 2.76 ´ ´ 10-23 when pH = 4.20 ´ (b) (ii) ´ In (b) (i), sulfide concentration calculated. In (b) (ii), common ion used to calculate [Fe2+] (error made). In (b) (ii), [Fe2+] calculated correctly. 10-5 mol L–1 1.10 ´ 10 –23 (10 ) –4.20 2 10-15 mol L–1 Ks = [Fe2+][S2-] 4.90 In (b) (i), [H3O+] calculated correctly 10–18 = [Fe2+] [Fe2+] = 1.78 ´ ´ 2.76 ´ 10–15 10-3 mol L-1 i.e., solubility of FeS is 1.78 4.20. ´ 10-3 mol L-1 when the pH of the solution is Excellence NCEA Level 3 Chemistry 91392 (3.6) — page 4 of 6 When hydrogen sulfide is bubbled through a solution containing Cu2+ and Zn2+ the following equilibrium is established. H2S (c) « 2H+ + S2- In (c), states the relationship between IP and Ks for precipitation to occur. As the pH of a saturated solution (by the addition of HCl) decreases, the equilibrium shifts in the reverse direction reducing [S2-]. In (c) links pH decrease to decrease in [S2–] using equilibrium principles. In (c), links solubility to a comparison of ionic product and solubility product for ZnS OR CuS. For precipitation to occur IP>Ks. Hence only the metal sulfide with the lowest Ks will precipitate. In this case CuS as IP(CuS)> Ks(CuS). The ZnS will not precipitate. As the pH then decreases [S2–] will increase. This enables metal sulfides with larger values of Ks in this case ZnS, to precipitate as well as the CuS. Not Achieved NØ No response; no relevant evidence. N1 Candidate provides some accurate statements without answering any question completely. N2 Candidate provides any ONE statement for Achievement. A3 Candidate provides any TWO statements for Achievement. A4 Candidate provides any THREE statements for Achievement. M5 Candidate provides any TWO statements for Merit. M6 Candidate provides any THREE statements for Merit. E7 Minor errors (eg omission or inaccuracies) from the Excellence criteria. E8 Candidate provides ALL the evidence from the Excellence criteria. Achievement Merit Excellence In (c), links pH decrease to decrease in [S2–] using equilibrium principles, AND links solubility to a comparison of ionic product and solubility product for BOTH ZnS and CuS. NCEA Level 3 Chemistry 91392 (3.6) — page 5 of 6 Three (a) (i) Expected coverage In this region CH3COOH and CH3COO– are both present. CH3COOH / CH3COO– is a conjugate acid/base pair. When base is added to the buffer system it will react with CH3COOH, thus maintain the pH by removing OH–. Achievement In (a) (i), recognises acid / base pair. Merit Excellence In (a) (i), links buffer to addition of base and includes equation OR CH3COOH + OH– CH3COO– + H2O X put on the graph at 10.00 mL In (a) (ii), X at 10mL with attempt at reason. in (a) (ii), links X at 10 mL to reason for most efficient buffer action. In b (i), [CH3COONa] correct. In (b) (i), method correct using incorrect [CH3COONa] (leading to error in pH 8.92). (a) (ii) The most efficient buffering occurs when the pH of the solution is equal to pKa ie [HA]=[A–]. Salt formed at equivalence point is CH3COONa. [CH3COONa] = 0.125/2 = 6.25 ´ 10-2 mol L-1. Ka = 10-4.76 = 1.74 ´ 10-5 CH3COOH + H2O CH3COO– + H3O+ [ H3O+] = (b) (i) = K a ´ Kw – [CH COO ] 3 1.74 ´10–5 ´1´10–14 6.26 ´10–2 = 1.67 ´ pH = 8.78 10–9 In (b) (i), pH shown to be 8.78 using correct value of salt concentration. NCEA Level 3 Chemistry 91392 (3.6) — page 6 of 6 (b) (ii) Phenolphthalein is a suitable indicator as its pKa is within 1 pH of equivalence point. Hence it will change colour at the equivalence point of the reaction in the steepest part of the graph. Methyl orange will change colour in the buffer region as it’s between pH 2.7 and 4.7 which is in the buffer region making this indicator unsuitable. Phenolphthalein is a weak acid and dissociates in water HIn + H2O (b) (iii) « H3O+ + In– In (b) (ii), limited reason given for use of phenolphthalein or nonuse of methyl orange. In (b) (ii), both indicators compared linked to reasons In (b) (iii), pH range of indicator given. In (b) (iii), link made between addition of acid or base to colour seen and pH range. When a base is added to the solution the equilibrium shifts in the forward direction. Therefore In- is purple. OR When acid is added the equilibrium shifts in the reverse direction, therefore HIn is colourless. Indicators are effective in the range pH = ± 1 pKa, ie between 8.60 and 10.6. Not Achieved NØ No response; no relevant evidence. N1 Candidate provides some accurate statements without answering any question completely. N2 Candidate provides any ONE statement for Achievement. A3 Candidate provides any TWO statements for Achievement. A4 Candidate provides any THREE statements for Achievement. M5 Candidate provides any TWO statements for Merit. M6 Candidate provides any THREE statements for Merit. E7 Candidate provides any ONE statement for Excellence. E8 Candidate provides BOTH statements for Excellence. Achievement Merit Excellence In (b) (iii), equilibrium principles used to explain addition of acid and of base linked to colour change and correct species over pH range.