BJDTCP3 and JDCAP Vet Manual [MS Word Document
advertisement

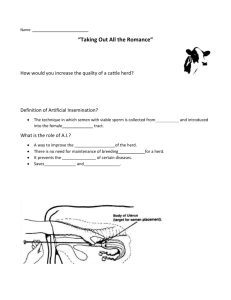
REF 9-01 Department of Environment and Primary Industries Victorian bovine Johne’s disease TCP3 and JDCAP Manual for Approved Veterinarians Contents Author: D Champness Version Date: 1 July 2013 Page 1 of 21 REF 9-01 Department of Environment and Primary Industries 1. 2. 3. 4. 5. 6. 7. 8. 9. DEPI Contact details for the TCP3 & JDCAP Programs Introduction TCP3 Program Rules Approved Veterinarian Responsibilities under the TCP3 Johne’s Disease Calf Accreditation Program (JDCAP) Investigation of Suspect Index clinical animals by Vet Practitioners Program Funding, Veterinary payments and Invoicing DEPI Definitions of Herd Prevalence level / Status Forms – TCP3 & JDCAP ACRONYMS APAV ASB bjd CattleMAP DEPI IN JDCAP NA PIC RD1 RD2 SU TCP TCP3 THP TLP TMP TMS Accredited Program for Australian Veterinarians Animal Standards Branch bovine Johne’s disease Cattle Market Assurance Program Department of Environment and Primary Industries Infected Herd Johne’s Disease Calf Accreditation Program Nil-Assurance or Non-Assessed Herd Property Identification Code Restricted 1 Restricted 2 Suspect Herd Test and Control Program Test and Control Program 3 Tested High Prevalence Tested Low Prevalence Tested Moderate Prevalence Tested to MAP Standard 1. DEPI Contact details for the TCP3 & JDCAP Programs All correspondence and administrative enquiries relating to the TCP3 and JDCAP should be sent to: TCP3 Admin Officer, Animal Standards Branch, DEPI Bendigo, PO Box 2500, Bendigo Delivery Centre, VIC 3554, Ph 54304507, Fax: 5430 4520. Email: tcp3admin@depi.vic.gov.au For technical enquiries contact: TCP3 Program Manager– Dr David Champness, DEPI Hamilton, Ph 55730703, Email: tcp3manager@depi.vic.gov.au Author: D Champness Version Date: 1 July 2013 Page 2 of 21 REF 9-01 Department of Environment and Primary Industries 2. Introduction The Victorian bovine Johne’s disease (bjd) Test and Control Program (TCP) is a voluntary program available for dairy and beef herds with a bjd infected status. The Johne’s Disease Calf Accreditation Program (JDCAP) is available to all dairy herds irrespective of their bjd status. As of 1st October 2010, the TCP program in its current form (TCP2) will cease and a revised program to be known as TCP3 will be introduced and administered by DEPI. Participation in the TCP3 program is voluntary. Participating herd owners / managers are required to comply with strategies to minimise bjd in their herds, engage their approved veterinarian to provide professional disease management advice and perform herd testing, and promptly dispose of test ELISA positive animals. Herds currently participating in the TCP2 must complete a new application in order to participate in the TCP3. Some herd owners currently participating in TCP2 may elect not to participate in TCP3, while some other infected herds not in TCP2 may decide to join the TCP3 program. Cattle herds participating in the TCP3 program must comply with the rules of the TCP3 program (refer to bjd TCP3 Program Rules in section 3). Under the TCP3 program, approved veterinarians will be required to provide professional advice on management strategies to minimise bjd in their client’s herds. This advice should be reinforced in writing to the herd owner / manager. Approved veterinarians will continue to be responsible for the collection and submission of herd test samples to the laboratory. Approved veterinarians will also be responsible for the reporting and NLIS identification of test positive animals, the development of the list of animals deemed Primary High-Risk and Secondary Risk of bjd, and the nomination of the herd bjd prevalence status. Section 4 outlines the responsibilities of the approved veterinarian including the reporting responsibilities to the herd owner and to the DEPI. Section 5 outlines the responsibilities of the approved veterinarian under the JDCAP program. The JDCAP is a calf-rearing program designed to minimise the risk of spreading Johne’s disease, should it be present, from adult cattle to replacement calves reared in the herd. The program requires the herd owner / manager, in consultation with their approved veterinarian, to establish a set of procedures for the rearing of replacement calves, document the steps to be taken, and monitor the program on an annual basis. Internal monitoring must identify when ‘short-cuts’ or omissions occur which jeopardise the integrity of the program and farmers must document the steps taken to overcome nonconforming practices. External auditing by approved veterinarians is conducted annually to provide confidence in the program for potential purchasers of cattle and to ensure the maintenance of program standards. Section 6 outlines the procedures for investigating suspect index clinical animals by veterinarians. Section 7 outlines the program funding and payments to veterinarians. Veterinary practitioners performing work under the TCP3 and JDCAP programs must be DEPI ‘approved veterinarians’. That is; have APAV accreditation, have attended a DEPI bjd TCP training session and have a current contract with DEPI. For more information contact Kim O’Neill at DEPI’s Bendigo office on Ph 54304507 or email - kim.o’neill@DEPI.vic.gov.au Author: D Champness Version Date: 1 July 2013 Page 3 of 21 REF 9-01 Department of Environment and Primary Industries 3. TCP3 Program Rules Victorian bovine Johne’s disease Test & Control Program (TCP3) Rules The Victorian bovine Johne’s disease (bjd) Test and Control Program (TCP3) is a voluntary disease control program operating with the support of the beef and dairy industries. It is administered by the Department of Environment and Primary Industries (DEPI). The TCP3 program is available to dairy and beef herds with a bjd infected status. Owners of herds participating in the TCP3 program must comply with the program rules as outlined below. General The herd owner / manager must engage a veterinarian approved by DEPI to provide professional advice on Johne’s disease control strategies and to conduct herd testing. A list of current DEPI approved veterinarians is available from DEPI on request. All dairy herds must have a current Certificate of Compliance issued by the veterinarian under the Johne’s Disease Calf Accreditation Program (JDCAP). Beef herds are exempt from participating in JDCAP. Herd Testing The ELISA serological test on a blood sample will be used for herd testing. An animal in a bjd infected herd that has a positive ELISA test result is a test positive animal and will be classified as a bjd infected animal. Animals that test positive to the ELISA serological test are not to be retested. Repeat serology is not permitted under the National bjd Standard Definitions and Rules with the exception of cases where the laboratory reports an ELISA test result as ‘suspect’, in which case the animal should be re-sampled a month later to determine the ‘status’ of the animal. Frequency of herd testing Herd tests are to be conducted either annually or every second year until the herd achieves Tested Low Prevalence (TLP). A herd of TLP status is an infected herd that has 2% or less test positive animals among cattle that are 4 years and older cattle. Once herds achieve TLP status (or better), a funded herd test will only be available every two years. TLP and Restricted 1 (RD1) herds will only require a herd test every two years. An RD1 status is allocated when the herd has achieved one negative herd test at least 12 months after the last known infected animal was removed from the herd. In consultation with their veterinarian, owners of TLP and RD1 herds may elect to test annually, but the funded testing will only be available every second year. Participation in the subsidised voluntary program may continue until the herd has achieved Restricted 2 (RD2) status. An RD2 status is achieved when all high risk animals have been removed from the herd and the herd has two consecutive negative herd tests two years apart. Until 1 September 2011, current TLP, RD1 and RD2 herds will be eligible to participate in one funded round of testing after which funded herd testing for TLP and RD1 herds will only be available every two years until the herd completes the program (or elects to withdraw from the program). Author: D Champness Version Date: 1 July 2013 Page 4 of 21 REF 9-01 Department of Environment and Primary Industries Age of cattle to be tested All cattle 4 years of age and older are to be tested. On the advice of the approved veterinarian, cattle less than 4 years of age (2 and 3 year olds) may be tested but this will be at the herd owner’s expense. Test Positive Animals The veterinarian must notify the herd owner / manager of the test results including (if any) a list of test positive animals. Notification to the herd owner / manager must be in writing within 14 days of the receipt of laboratory results. The veterinarian is responsible for identifying the test positive animals, including the individual NLIS tag details of these. The veterinarian must report NLIS tag details of the test positive animals to the DEPI. There is no longer a requirement to ear tag test positive cattle with a yellow DEPI ‘arrow’ ear tag. Cattle which are test positive must be disposed within 30 days of receipt of test results from the veterinarian. These test positive cattle must be disposed for slaughter only direct to an abattoir, knackery, or on-farm. Cattle showing signs consistent with Johne’s disease must not be sent to an abattoir for slaughter for human consumption; they must be either disposed direct to a knackery or disposed on-farm. Herd owners must dispose of test positive animals within the 30 day timeframe to continue to be eligible for subsidised testing. Failure to comply with disposal of test positive animals in a timely manner jeopardises onfarm disease control. DEPI will monitor the NLIS database for compliance with this requirement. Disregard for this requirement will lead to denied access to the funded TCP3 program. The NLIS details of any cattle disposed on-farm must be updated on the NLIS database to reflect the ‘deceased’ status of the animal’s NLIS tag. High-Risk animal list A list of animals deemed primary high-risk and secondary risk of Johne’s disease will be developed by the approved veterinarian in consultation with the herd owner / manager based on herd progeny records. Primary High-Risk - animals deemed primary high-risk of bjd include: the dam and all remaining progeny of clinical animals, the dam and immediate progeny (latest calf) of test positive animals, and any other animals the veterinarian considers to be primary high-risk. Secondary Risk - additional animals which may be deemed a secondary risk of bjd include: any other progeny of test positive animals not included in the primary high-risk list, animals raised as the cohort age group of an animal/s with clinical bjd. Any cattle remaining in the herd which were on the previous Preferential Cull List are to be transferred to the High-Risk animal list on commencement of participation in TCP3. Animals identified by the veterinarian as primary high-risk must be removed from the herd within 12 months and disposed by slaughter only. Animals deemed at secondary risk must be disposed from the herd by slaughter only. There is no time limit on the disposal of animals on the secondary risk list. Primary high-risk Author: D Champness Version Date: 1 July 2013 Page 5 of 21 REF 9-01 Department of Environment and Primary Industries and secondary risk animals can only be disposed directly to an abattoir, knackery, or on-farm. They must not enter a public place, such as a saleyard or scales operation. Cattle showing clinical signs consistent with Johne’s disease cannot be sent to an abattoir for slaughter for human consumption; they must be disposed either direct to a knackery or disposed on-farm. Cattle with clinical signs consistent with or suspicious of bovine Johne’s disease Under Victorian legislation, a herd owner / manager must notify DEPI within 7 days of the owner becoming aware of cases of bjd or suspicion of bjd in their herd. Any animals with clinical signs consistent or suspicious of bjd (gradual weight loss / emaciation and chronic diarrhoea which is unresponsive to treatment) must be notified to DEPI and the veterinarian. Notification to DEPI can be either by way of a fax or email addressed to the TCP3 Admin Officer - Fax 03 5430 4520, or email: tcp3admin@DEPI.vic.gov.au Details of this notification must include the herd owner / manager’s name, Property Identification Code (PIC), the number and age of ‘clinical’ animals and the date of onset of signs. The veterinarian should give you professional advice on an appropriate course of action for any clinically affected animals. To minimise further contamination of the property with the Johne’s bacteria and to minimise animal welfare issues, cattle displaying signs consistent with bjd should be removed from the herd as soon as possible and disposed of. These animals cannot be sent to a saleyard or abattoir. They must be disposed of through a knackery or on-farm in an appropriate and humane manner. There is no obligation to investigate these ‘clinical’ animals, other than notification to DEPI and prompt disposal of the animal. The herd owner / manager and veterinarian may elect to sample the animal to confirm bjd or test for another disease. Any laboratory fees for the testing for bjd in these cases will be paid by DEPI directly to the laboratory. The herd owner will be responsible for the veterinary fees associated with any consultation and/or sampling and any other (non-bjd related) laboratory fees. Silirum vaccinated cattle Any cattle which have been vaccinated with the bjd vaccine – Silirum™ and test serological (ELISA) positive are deemed test positive animals and are to be treated the same as non-vaccinated test positive animals. Any progeny of a ‘Silirum vaccinated’ clinical case cow should be added to the Primary High-Risk animal list to be disposed for slaughter only within 12 months as per other the Primary High-Risk listed animals. Progeny of any ‘Silirum vaccinated’ test positive cows should be added to the Secondary Risk animal list to be disposed for slaughter only. This policy on Silirum vaccinated cattle is based on a conservative approach to risk until more data becomes available on the vaccine efficacy. Introducing cattle into the herd The dairy herd owner / manager may only introduce cattle into the herd that have the equivalent or higher National Dairy Assurance Score (NDAS). For example a dairy herd with a Restricted 1 status (NDAS = 5 points) could introduce dairy cattle from a herd with a Tested Low Prevalence status (NDAS = 4 points) if the calves were raised under the 3-Step Calf Plan which entitles them to 1 calf credit point, totalling 5 Points. Calves raised under the audited JDCAP are entitled to 3 calf credit points. Author: D Champness Version Date: 1 July 2013 Page 6 of 21 REF 9-01 Department of Environment and Primary Industries Dairy herds should obtain / retain a copy of a Dairy bjd Assurance Score Declaration form or a National Vendor Declaration (NVD) which states the National Dairy Assurance Score, for any cattle purchased. Beef herds may only introduce cattle into the herd which originate from herds certified to be of equal or better level of assurance (status) in relation to Johne’s disease. For example a beef cattle herd with a status of RD2 must not introduce cattle from a herd which is only RD1. Herd owners / managers must ensure that the NLIS database is notified within 7 days of the introduction of cattle including bulls directly from another property with a different PIC. Johne’s Disease Calf Accreditation Program Dairy herds participating in the TCP3 must continue to comply with the requirements of the JDCAP and have a current Certificate of Compliance. Participating herds that are issued with a Provisional Certificate of Compliance must address and rectify noncompliances and obtain a Certificate of Compliance within 12 months of receiving the Provisional Certificate. Program Funding / Cost of Program The TCP3 program is funded by the Victorian Cattle Compensation Fund with monies generated from livestock duties. The Cattle Compensation Advisory Committee, appointed by the Minister of Agriculture from representative cattle industry groups, is responsible for advising the Minister on expenditure from the Fund for livestock disease control programs. Subsidisation of the funded program administered by DEPI will be limited to the agreed fee payment for: Veterinary fees for the collection and submission of herd samples from animals 4 years and older and the required veterinary activity following herd testing. This includes the written notification of herd test results to the herd owner / manager, the recommended herd status, the reporting of the individual NLIS identification of test positive animals to DEPI, and in consultation with the herd owner / manager, the development of a written list of animals deemed high-risk of Johne’s disease. Annual veterinary fee for advice to the herd owner / manager relating to Johne’s disease control, the annual updating of the list of high-risk animals and the supply of the written annual report to DEPI. Laboratory cost for annual / biennial (as applicable) herd testing of animals 4 years and older. (Cost for testing cattle less than 4 years old will be at the owner’s expense). Laboratory cost for confirmation of Johne’s disease in any clinical animals sampled. These veterinary fees are payable by DEPI directly to the approved veterinarian upon receipt of a completed report and invoice to DEPI from the veterinarian. DEPI will directly pay the laboratory fees for testing as described above. The applicant (herd owner / manager) will be responsible for payment to the engaged veterinarian for any fees over and above the funded fee. Herd owners / managers should discuss their individual circumstances with their veterinarian and whether there is likely to be any additional veterinary fees above the funded program fees. Author: D Champness Version Date: 1 July 2013 Page 7 of 21 REF 9-01 Department of Environment and Primary Industries Veterinary fees for the annual JDCAP audit are payable by the herd owner. The herd owner may claim from DEPI, up to the maximum rebate (currently $250) upon lodgement of a JDCAP claim form along with a copy of the veterinarian’s invoice and receipt as proof of payment. Compensation Under the TCP3 program, no compensation will be available for clinical animals or serological (ELISA) test positive animals. TCP3 Program commencement date The TCP3 program will commence from the 1st October 2010. It supersedes the current TCP2 program. The TCP3 program will be reviewed mid 2011 and some conditions associate with the operation of the program may change after consultation with industry stakeholders and with the endorsement of the Cattle Compensation Advisory Committee. Application to participate in the TCP3 Program In order to participate in this subsidised voluntary program, the herd owner / manager (applicant) is required to complete an application form acknowledging they understand and will comply with the rules of the program. The application form must be completed for herds currently participating in the TCP2 program and wishing to continue in the new TCP3 program, along with any new herds wishing to join the TCP3. TCP3 Program Flow Chart Below is a flow chart depicting the frequency of testing and herd progression through the TCP3. Author: D Champness Version Date: 1 July 2013 Page 8 of 21 REF 9-01 Department of Environment and Primary Industries Diagram outlining herd progression through the TCP3 Testing Frequency A n n u a l Infected Herd No Herd Test ≤2% test positive animals Positive Yes Tested Low Prevalence No Herd Test Positive B i e n n i a l Meets criteria (Note 1) Negative Negative NB Note 1 Yes Restricted 1 (RD1) Herd Test Positive Negative Restricted 2 (RD2) TCP3 ceases Key and Notes Status progression - to progress to a higher status, all of the animals on the Primary High-Risk list must have been removed from the herd and disposed of for slaughter only. Test positive animals must be removed for slaughter only within 30 days Clinical cases – occurrences of cases in RD1 or RD2 herds will result in the herd being reclassified as Tested Low Prevalence (TLP), High-risk animal lists to be updated Note 1 – To be eligible for RD1, at least 12 months must have passed since the last clinical case was removed (in addition to meeting the ‘Status progression’ note above. Author: D Champness Version Date: 1 July 2013 Page 9 of 21 REF 9-01 Department of Environment and Primary Industries 4. Approved Veterinarian Responsibilities under the TCP3 Approved veterinarians will be more directly involved in the TCP3 than was previously the case. DEPI Animal Health field staff will no longer be involved with the identification of test positive animals or the development of the list of high-risk animals. This will now be the responsibility of the consulting veterinarian. Under the TCP3 program, the approved veterinarian will be responsible for: 4.1. Herd Management Advice This will be professional advice to the herd owner / manager on management strategies to: minimise the impact of Johne’s disease on the dairy or beef herd operation, minimise the contamination of the farm environment, farm biosecurity measures to minimise the risk of introduction of further JD infected animals into the herd such as through the use of the National Dairy bjd Assurance Score, and advice on the timely recognition, diagnosis and immediate disposal of clinical animals. This advice should be reinforced regularly and in writing, at least annually when the herd test is conducted and / or during the annual JDCAP audit. Review of the High-Risk animal list should inform the development of advice for improvements to herd management. Under the TCP3, approved veterinarians will be paid two hundred dollars (inclusive of GST) upon receipt by DEPI of an invoice and a completed annual report. The required report forms are within the appendices of this manual. Refer to section 9 for a list of forms. Bjd TCP3 Annual / Biennial Herd Test Veterinary Report Form (F9-04) is to be used for the annual reporting in years that a herd test in conducted. Bjd TCP3 Annual Herd Veterinary Report Form (F9-05) is to be used for the annual reporting in those years in which herd testing is not conducted. 4.2. Herd Testing 4.2.1. Age of animals to test Cattle 4 years and older are to be sampled. In consultation with the herd owner / manager and on the advice of the veterinarian, cattle less than 4 years of age (2 and 3 year olds) may be tested but this will be at the owner’s expense. Submission of the blood samples is to an approved laboratory (currently Gribbles Veterinary Laboratory) for serological JD ELISA testing. 4.2.2. Frequency of Herd Testing Herd testing of cattle 4 years of age and older is to be conducted either annually or every second year until the herd achieves Tested Low Prevalence (TLP) as determined by the approved veterinarian. Once a herd achieves TLP status (or better), a herd test will only be funded every second year until RD2 status is reached. TLP and Restricted 1 (RD1) herds will only require a herd test every two years. In consultation with their approved veterinarian, herd owners may elect to test annually, but the funded testing will only be available every two years. Refer to section 8 for the definitions of TLP and RD1. Until 1 September 2011, current TLP, RD1 and RD2 herds will be eligible to participate in one funded round of testing, after which funded herd testing for TLP and RD1 herds will only be available every two years until the herd completes the program (or elects to withdraw from the program). Author: D Champness Version Date: 1 July 2013 Page 10 of 21 REF 9-01 Department of Environment and Primary Industries 4.3. Identification of Test Positive Animals The approved veterinarian is responsible for the identification of serological (ELISA) positive animals including their individual NLIS Identification. The NLIS details of test positive animals must be reported to the DEPI on the annual report (see veterinary reports in section 4.7 below). There is no longer a requirement to ear tag test positive cattle with a yellow DEPI ‘arrow’ ear tag. However herd owners / managers may wish to use some easily visible form of identification such as a coloured ear tag or paint mark until these animals are disposed of from the herd. It is preferable to scan the NLIS RFID tags of test positive animals as this prevents transcription errors which may occur with recording the external NLIS number. If NLIS tags of test positive animals are scanned, the download file can be emailed to the TCP3 Admin Officer (refer to section 1 for email address). The email should state the herd owner and PIC. For herds which have all animals NLIS tagged, vets may choose to utilise the NLIS technology – scanning cattle at the time of sample collection. This scanning data can be used to produce the ‘bleed sheet’ for lab submissions, connected to a label printer to print blood tube labels, and referred to later for easy NLIS identification of test positive animals. 4.4. ELISA Suspect test result Current ELISA test kits used by Gribbles laboratory have an amended manufacturer’s protocol which now includes a ‘suspect’ zone between the cut off point for a positive or negative result. These are reported as a ‘suspect’ test result. In cases where the laboratory reports an ELISA test result as ‘suspect’, the animal should be resampled a month later to determine the ‘status’ of the animal. In consultation with the approved veterinarian, the herd owner may choose to dispose of the ‘suspect’ test result animal (for slaughter only) without re-sampling. There is no requirement to add the dam and any progeny of a ‘suspect’ test animal to the high-risk animal list, unless the animal is confirmed positive on a re-sample test a month later. 4.5. High-Risk Animal list In consultation with the herd owner / manager, the approved veterinarian must develop a list of Primary High-Risk and Secondary Risk animals (previously known as the Preferential Cull List). Any cattle remaining in the herd which were on the previous Preferential Cull List must be transferred to the High-Risk animal list. Primary High-Risk - as a minimum, animals deemed Primary High-Risk of bjd include: the dam and all remaining progeny of clinical animals, the dam and immediate progeny (latest calf) of test positive animals, and any other animals the veterinarian considers to be high-risk. Secondary Risk - additional animals which may be deemed a Secondary Risk of bjd include: any other progeny of test positive animals not included in the primary high-risk list, and animals raised as the cohort age group of an animal/s with clinical bjd. The herd owner / manager must be given a copy of this list and a copy must be attached to the annual report to DEPI (see reporting section 4.7 below). Author: D Champness Version Date: 1 July 2013 Page 11 of 21 REF 9-01 Department of Environment and Primary Industries Animals identified as Primary High-Risk must be removed from the herd and disposed by slaughter only within 12 months. Animals deemed as Secondary Risk must also be disposed from the herd by slaughter only. There is no time limit on the disposal of animals on the secondary risk list. These primary high-risk and secondary risk animals may be disposed of to an abattoir, knackery, or on-farm. Cattle showing clinical signs consistent with Johne’s disease must not be sent to an abattoir for slaughter for human consumption; they must be disposed either direct to a knackery or disposed on-farm. Review of the High-Risk animal list should inform the development of advice for improvements to herd management as outlined in section 4.1. 4.6. Silirum Vaccinated Cattle Any cattle which have been vaccinated with the bjd vaccine – Silirum™ and test serological (ELISA) positive are deemed test positive animals and are to be treated the same as non-vaccinated test positive animals. Any progeny of a Silirum vaccinated clinical case cow should be added to the Primary High-Risk animal list to be disposed for slaughter within 12 months as per other Primary High-Risk listed animals. Progeny of any Silirum vaccinated test positive cows should be added to the Secondary Risk animal list to be disposed for slaughter only. This policy on Silirum vaccinated cattle is based on a conservative approach to risk until more data becomes available on the vaccine efficacy. 4.7. Herd disease prevalence level / Status The approved veterinarian is responsible for determining the disease prevalence level or status of the herd such as Tested Low Prevalence, Moderate or High Prevalence, RD1 or RD2 status. Refer to section 8 for a list of herd prevalence levels / status definitions. The herd owner / manager must be advised in writing of the herd prevalence level / status as determined by the veterinarian following each herd test (Farmer Report form F9-02). This status information must also be included on the annual veterinary report. 4.8. Veterinary reports to herd owners and DEPI Refer to section 9 for a list of report forms and proformas to herd owner / manager. 4.8.1. Notification / Report to the herd owner / manager The approved veterinarian must within 14 days of receipt of laboratory results, report the test results in writing to the herd owner / manager including a list of test positive animals (if any) for culling (disposal by slaughter within 30 days of receipt of vet report). The DEPI proforma bjd TCP3_Farmer Report Form (F9-02) is sufficient to meet these requirements. A copy of this farmer report form must be sent to DEPI with the annual herd veterinary report. 4.8.2. Annual Herd Veterinary Reports to DEPI A completed annual herd veterinary report must be supplied to the DEPI - TCP3 Program Manager - within 30 days of reporting test results to the farmer. Refer to section 9 for a list of report documents. The Annual / Biennial Herd Test Veterinary Report (F9-04) is to be used in the year of a herd test. The Annual Herd Veterinary Report (non-test year) (F9-5) is to be used in years in which there is no herd test conducted. These reports must include among other things, details of the herd test including the date of testing, lab submission number, number of samples ( >4yo and 2-3 year olds if any), number of test ELISA positives with Author: D Champness Version Date: 1 July 2013 Page 12 of 21 REF 9-01 Department of Environment and Primary Industries a list of their individual NLIS details, the number of clinical animals in the previous 12 months, Prevalence level / status (eg TLP), and the date the herd owner / manager was notified of the test results. The list of HighRisk animals must be attached. As stated in section 4.1, the herd management advice should be reinforced in writing to the herd owner / manager. A copy of this written herd management advice should be sent to DEPI with the annual reports. Author: D Champness Version Date: 1 July 2013 Page 13 of 21 REF 9-01 Department of Environment and Primary Industries 5. Johne’s Disease Calf Accreditation Program (JDCAP) Participation in the Johne’s Disease Calf Accreditation Program (JDCAP) remains available to all dairy herds irrespective of their bjd status. As per TCP2, dairy herds wishing to participate in TCP3 are required to enrol in the JDCAP and hold a current Certificate of Compliance. A Provisional Certificate of Compliance is acceptable for the first 12 months. Beef herds participating in TCP3 are exempt from participation in JDCAP. It is the responsibility of the herd owner / manager to ensure that an audit (review) is conducted annually (within 10 to 14 months of the last audit) by their approved veterinarian. Ideally the audit should be undertaken when the calves are being reared. The task may be done in conjunction with a farm visit for some other purpose provided the herd owner / manager agrees to the audit at that time. To ensure integrity and consistency in the implementation of the JDCAP program, some important details and changes are outlined below. Refer to the JDCAP Manual for full details of the program. 5.1. Farm Documentation The minimum documentation for JDCAP is: A calf rearing plan that addresses all the elements of JDCAP. A farm map showing paddocks, effluent drainage and any significant features of the farm. Herd breeding records showing calf birth dates, dam and permanent identification. These records may be in the form of a computer database, paper or card records, or a ‘shed sheet’ or any other format that allows data storage and retrieval for the whole lifetime of the calf/cow herd. The signed JDCAP agreement made between the herd owner and an approved veterinarian. Additional documentation may be required: Any variation to the calf rearing plan. An incident reporting form or something similar Records of animal purchases. These are required if calves under the age of 12 months are introduced into the JDCAP group. The records should include the vendor’s details and a National bjd Dairy Assurance Score declaration or bjd Animal Health Statement indicating that the introduced calves were born and reared under JDCAP or CattleMAP. Records of agistment. These are required if JDCAP calves under the age of 12 months are agisted on another property, or are new calves introduced into the JDCAP group for agistment. The records should include details of the other calves’ owner and a National bjd Dairy Assurance Score declaration or bjd Animal Health Statement indicating that the introduced calves were born and reared under JDCAP or CattleMAP. 5.2. On-farm – System Monitoring Regular monitoring of the calf rearing plan should be undertaken by the person primarily responsible for the calf rearing. Any incidents resulting in breeches of calf/cow separation should be recorded along with the corrective action that was undertaken. Likewise, any alterations to the agreed plan should be noted. A dedicated JDCAP diary or a note similar to the incident report form, are suitable for this purpose. Events should be recorded within 48 hours of their occurrence. The incident report form should be reviewed by the farm’s approved veterinarian on their next visit to the property. The approved veterinarian should initial each event line to indicate that it has been discussed and the future management of the calves has been reviewed. 5.3. Annual JDCAP Audit (Review) - Responsibilities of the approved veterinarian As part of the annual audit (review), the approved veterinarian must: Visit the property and examine the calf rearing facilities and paddocks grazed by weaned calves. Author: D Champness Version Date: 1 July 2013 Page 14 of 21 REF 9-01 Department of Environment and Primary Industries Ensure that accurate and complete records are kept by the herd owner / manager (see 5.1 above). Complete a JDCAP Annual Property Review Summary form. A copy of this completed form must be forwarded to DEPI (TCP3 Admin Officer) within 14 days of the audit. With the farmer, address non-compliance with the agreed program o Minor non-compliance with the program will require corrective actions to be taken. The vet should complete a JDCAP Corrective Action Report if required and ensure that a realistic timeframe is set for the corrective action to be completed. The herd owner / manager must sign the bottom of the Corrective Action Report form and receive a copy. The approved veterinarian must ensure that any required ‘corrective actions’ are resolved by the next annual audit. A copy of this report should be forwarded to DEPI. o Major non-compliance may result in loss of accreditation. Any major non-compliance should be reported to the TCP3 Program Manager. Update the calf rearing plan with the herd owner as necessary. Issue an updated JDCAP Certificate of Compliance (see 5.3.1 below). 5.3.1. Issuing of a Certificate of Compliance / Provisional Certificate of Compliance Certificate Numbers for new herds will be issued to the approved veterinarian by the TCP3 Admin Officer. Refer to contact details in section 1 of this manual. Issuing Certificates of Compliance - It is the responsibility of the approved veterinarian to issue as appropriate a Certificate of Compliance or a Provisional Certificate of Compliance to the herd owner / manager. Provisional certificates may only be issued in the first year of participation. DEPI does not issue these certificates. A copy of the certificate must be forwarded to DEPI (TCP3 Admin Officer) within 14 days of issue. If assistance is required, contact the TCP3 Admin Officer. 5.4. Herd Owner Rebate To access these rebates, herd owners / managers must submit a completed claim form to DEPI (TCP3 Admin Officer), with a copy of the veterinary practice invoice / receipt and a copy of the JDCAP Certificate of Compliance. 5.4.1. Initial JDCAP Herd Plan rebate The rebate available for dairy farmers for the initial JDCAP herd plan and report remains at a maximum of $356 (inclusive of GST). 5.4.2. Annual JDCAP Audit rebate From 1st October 2010, the annual JDCAP audit rebate available to dairy farmers will increase to a maximum of $250 (inclusive of GST) per annum. 5.5. Administration of JDCAP and Reporting to DEPI JDCAP will now be administered centrally by Animal Standards Branch (ASB) at DEPI Bendigo, rather than through regional Senior Veterinary Officers. From now on, please send JDCAP correspondence directly to the TCP3 Admin Officer, Animals Standards Branch, DEPI Bendigo. See contact details in section 1 of this manual. A copy of all reports and certificates issued by the approved veterinarian must be forwarded to the TCP3 Admin Officer. Author: D Champness Version Date: 1 July 2013 Page 15 of 21 REF 9-01 Department of Environment and Primary Industries 6. Investigation of Suspect and Index Johne’s disease cases by Veterinary Practitioners Bovine’s Johne’s disease (bjd) is a notifiable disease under the Livestock Disease Control Act 1994. Livestock owners and veterinarians have an obligation to notify DEPI of suspicion of Johne’s disease within 7 days of the owner becoming aware of cases or suspicion of bjd. Where a producer notifies DEPI directly, DEPI will refer the producer to their private veterinarian to provide advice on the appropriate course of action including any further investigation of the case. Herds which do not have a history of bjd (Non-Assessed herds) which have animals reported with clinical signs suspicious of bjd should be investigated as per section 6.1 (below) to confirm or exclude Johne’s disease. Herds participating in the TCP3 program which have animals reported with clinical signs suspicious of bjd should be given advice on an appropriate course of action as per section 6.2 (below). 6.1. Investigating a suspect ‘Index’ case on a property with no history of JD (NA herds) In herds in which bjd has not been diagnosed previously (Non-Assessed herds), animals displaying clinical signs suspicious or consistent with Johne’s disease should be investigated. As a minimum, the approved veterinarian should submit a blood sample for serological (ELISA) testing and a faecal sample for culture for JD to Gribbles Veterinary Laboratory. These tests to confirm or exclude bjd (a notifiable disease) will be invoiced directly to DEPI by Gribbles. The approved veterinarian may elect to necropsy the clinical animal on-farm or at a knackery to assist in the diagnosis of the causative disease. Where the standard set of tissue samples (as per the CattleMAP Manual) are submitted for histopathology and/or culture, the cost of this laboratory testing can be invoiced directly to DEPI by Gribbles. Refer to section 6.4 (below) for payments to veterinary practitioners for these suspect / index case investigations. Any veterinary fee above the DEPI Significant Disease Investigation (SDI) payment such as an additional fee for necropsy examination by the practitioner will be borne by the herd owner / manager. Where a test positive result occurs (ELISA or faecal culture positive), the approved veterinarian must give the herd owner / manager advice on management strategies for Johne’s disease and the TCP3 and & JDCAP programs. 6.2. Course of action for clinical animals in TCP3 herds The approved veterinarian should provide professional advice for any clinically affected animals. This includes confirming the suspicion of clinical bjd and where appropriate providing advice on the prompt disposal of the animal to minimise any animal welfare issues and minimise further contamination of the property with Mptb bacterium. These clinical animals must not be sent to a saleyard or abattoir. They must be disposed through a knackery or on-farm in an appropriate and humane manner. Under the TCP3 program, there is no further obligation by the herd owner / manager to investigate these ‘clinical’ animals, other than to notify to DEPI and their approved veterinarian and promptly dispose of the animal. As the approved veterinarian, in consultation with the herd owner / manager, you may elect to sample the animal to confirm bjd or test for another disease. Any laboratory fees for appropriate samples sent to Gribbles Veterinary Laboratory, such as blood for an ELISA test or a faecal sample for culture for the confirmatory testing for bjd in these cases will be paid by DEPI directly to the laboratory. The herd owner Author: D Champness Version Date: 1 July 2013 Page 16 of 21 REF 9-01 Department of Environment and Primary Industries will be responsible for the veterinary fees associated with any consultation and/or sampling and any other (non-bjd related) laboratory fees. The veterinarian should record the identification details of any reported clinical animals to enable the updating of the Primary High-Risk animal list, and the annual herd veterinary report to DEPI. 6.3. Reporting of investigations to DEPI Approved veterinarians should report investigations of suspect ‘Index’ cases on a Record of Disease Event (RODE) report form with a copy of any laboratory reports sent to the TCP3 Admin Officer (see section 1 for contact details). Reports and investigations of clinical cases in TCP3 participating herds should be reported on the TCP3 annual herd veterinary report. 6.4. Payment to veterinarians Veterinarians may invoice DEPI ($330 inclusive of GST) for the ‘Index case’ investigations outlined in section 6.1 (above) as per the Significant Disease Investigation regime with the completion of a RODE report form and invoice forwarded to the TCP3 Admin Officer (see section 1 for contact details). Author: D Champness Version Date: 1 July 2013 Page 17 of 21 REF 9-01 Department of Environment and Primary Industries 7. TCP3 Program Funding, Veterinary Payments and Invoicing DEPI 7.1. Program Funding The TCP3 and JDCAP programs are funded from the Cattle Compensation Fund under the recommendation of the Cattle Compensation Advisory Committee to the Minister of Agriculture. DEPI is responsible for the administration of these programs. The program funding has been set by the Cattle Compensation Advisory Committee with consideration to the sustainability of the fund and on the advice of DEPI based on the ‘average’ dairy herd size and average veterinary fees. Veterinarians may elect to charge their clients additional veterinary fees above the TCP3 funded fees and/or the JDCAP maximum rebate. The herd owner / manager will be responsible for payment to the veterinarian for any fees above the funded fees. Veterinarians should be up front with their clients and discuss the possibility of additional veterinary fees and under what circumstances these additional fees may be charged. This discussion should take place before any program work is undertaken, preferably when a herd owner / manager first contacts you as a DEPI approved veterinarian. Subsidisation of the funded program will be limited to payment of the veterinary and laboratory fees and rebates listed below: 7.2. Veterinary Fees 7.2.1. Annual Veterinary Consultation Fee An annual Veterinary Consultation Fee of $200 (inclusive of GST) will be paid upon invoice to DEPI for advice to the herd owner / manager relating to Johne’s disease control, the annual updating of the list of highrisk animals and the supply of the written annual herd veterinary report to DEPI. Refer to section 4.7.2 for the required annual reporting to DEPI. 7.2.2. Veterinary Fee (herd sampling and follow-up work) A Veterinary Fee of $6.35 (inclusive of GST) per animal sampled will be paid for the collection and submission of herd samples from animals 4 years of age and older and the required veterinary activity following herd testing. This includes the written notification of herd test results to the herd owner / manager, the recommended herd status, the reporting of the individual NLIS identification of ELISA test positive animals to DEPI, and in consultation with the herd owner / manager, the development of a written list of animals deemed primary high-risk and secondary risk of Johne’s disease. The funded fee of $6.35 per animal sampled is an additional $1.10 above the 2009/10 TCP2 fee of $5.25. This additional amount is to cover the additional activity required following herd testing 7.3. Laboratory Fees 7.3.1. Herd testing laboratory fees Laboratory fees for annual / biennial (as applicable) serological (ELISA) herd testing of animals 4 years of age and older will be funded under the program. Gribbles veterinary laboratory will invoice DEPI directly for TCP3 eligible herd testing. The laboratory fees for testing 2 and 3 year old animals are not covered under the funded program. If the veterinarian elects to test 2 and 3 year old animals, these additional lab fees will be at the owner’s expense. It is the responsibility of the veterinarian to pay Gribbles for any additional tests and recoup this from the herd owner. Author: D Champness Version Date: 1 July 2013 Page 18 of 21 REF 9-01 Department of Environment and Primary Industries 7.3.2. Suspect Index case and clinical animal lab fees Laboratory fees related to bjd testing through Gribbles veterinary laboratory for investigation of Suspect Index bjd cases in non-assessed herds and of clinical animals in TCP3 herds, will be funded under the program. Approved veterinarians should submit a blood sample for serological (ELISA) test and/or a faecal sample for culture to confirm Johne’s disease in a suspect (index case) or clinical animal. The approved veterinarian may also submit a set of standard tissue samples (as per CattleMAP Manual) for histopathology and culture to confirm an index case or clinical animal in a TCP3 herd. 7.4. Further testing of ELISA Positive or Clinical Animals in TCP3 Herds 7.4.1. Clinical Animals in TCP3 herds As outlined in section 3, Rules of the TCP3 program; there is no obligation to further investigate bjd clinical animals in TCP3 herds by sampling and laboratory testing. The veterinarian in consultation with their client (herd owner / manager) may elect to further investigate these clinical animals such as by serology (ELISA) or faecal sample for culture or necropsy with collection of appropriate tissue samples. Any veterinary fees relating to this investigation and sampling will be at the herd owner’s expense. The program will fund the laboratory fees related to bjd testing where appropriate samples are submitted to Gribbles veterinary laboratory. 7.4.2. ELISA Positive Animals in TCP3 herds Animals which test positive to the ELISA serological test are not to be re-tested. Repeat serology is not permitted under the national bjd SDRs. As outlined in section 3, Rules of the TCP3 program; there is no obligation to further investigate by sampling any ELISA positive animals. The program will not fund any veterinary or laboratory fees relating to further sampling of ELISA positive animals. 7.5. JDCAP Rebate to farmers 7.5.1. Initial JDCAP plan and report rebate The initial JDCAP plan and report rebate remains at a maximum of $356. 7.5.2. Subsequent Annual JDCAP audit rebate JDCAP annual audit rebate available to dairy farmers has increased to a maximum of $250 (was $237 in 2009/10). 7.6. Veterinary Fees paid by DEPI outside the funded program fees 7.6.1. Significant Disease Investigation fee A Veterinary consultation fee equivalent to the Significant Disease Investigation / Record of Disease Event (RODE) report fee ($330 inclusive of GST) will be paid upon invoice to DEPI where the veterinarian investigates a suspect clinical index JD case (as outlined in section 6 of this manual) and gives advice to the herd owner / manager on management strategies for Johne’s disease, the TCP3 and JDCAP programs. 7.7. Invoicing DEPI Tax Invoices to DEPI are to be sent to the TCP3 Admin Officer (refer to section 1 for TCP3 Admin Officer contact details). To ensure quick payment, copies of all relevant reports, should be sent at the same time as the invoice. Author: D Champness Version Date: 1 July 2013 Page 19 of 21 REF 9-01 Department of Environment and Primary Industries 8. Herd Prevalence level / Status Definitions Based on the National bjd Standard Definitions and Rules, these definitions have been adopted by the dairy industry as per the Dairy bjd Assurance Score Declaration form. These prevalence level statuses are applicable for herds that are participating in an approved bjd control program. Tested Low Prevalence (TLP): An Infected herd that has 2% or less ELISA positives among cattle that are 4 years and older (or 1.5% or less in cattle 2 years and older). Tested Moderate Prevalence (TMP): An Infected herd that has equal or less than 4% test positive by ELISA among cattle that are 4 years and older (or 3% or less in cattle 2 years and older). Test High Prevalence (THP): An Infected herd that has more than 4% of cattle 4 years and older test positive by ELISA (> 3% of 2 cattle years and older) or have not been tested. Restricted 1 (RD1): RD1 status is allocated when the herd has achieved one negative herd test at least 12 months after the last known infected animal was removed from the herd. If further testing is not completed after two years, the herd will revert to a status of Infected Restricted 2 (RD2): RD2 status is allocated when the herd has achieved two (consecutive) negative herd tests two years apart with the first negative test at least 12 months after the last known infected animal was removed from the herd. If further testing is not completed after two years, the herd will revert to a status of Infected. Author: D Champness Version Date: 1 July 2013 Page 20 of 21 REF 9-01 Department of Environment and Primary Industries 9. Forms All of these forms are available in Microsoft word format. Some are also available as an electronic word version. The electronic format forms can be emailed upon request. Contact the TCP3 Admin Officer. 9.1. TCP3 Forms Application to enrol in TCP3 form (F9-01) Farmer Report form (F9-02) List of Animals deemed Primary High-Risk and Secondary Risk of bjd (F9-03) Annual / Biennial Herd Test Veterinary Report form (F9-04) Annual Herd Veterinary Report form (non-test year) (F9-05) 9.2. JDCAP Forms JDCAP Annual Property Review Summary (F9-06) JDCAP Certificate of Compliance (F9-07) JDCAP Provisional Certificate of Compliance (F9-08) JDCAP Corrective Action Report (F9-09) JDCAP Incident report form (F9-10) JDCAP Claim form (F9-11) JDCAP Application for inclusion in the public register of herds (F9-12) Author: D Champness Version Date: 1 July 2013 Page 21 of 21