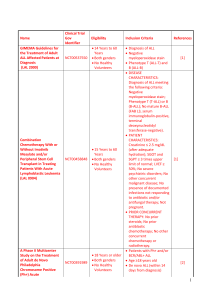
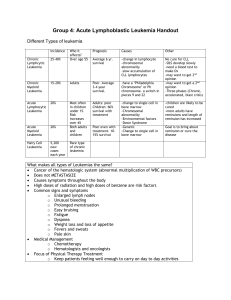
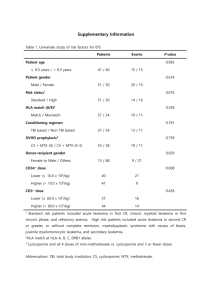
Supplement 2 Candidate risk factors for the study Comorbidity
advertisement
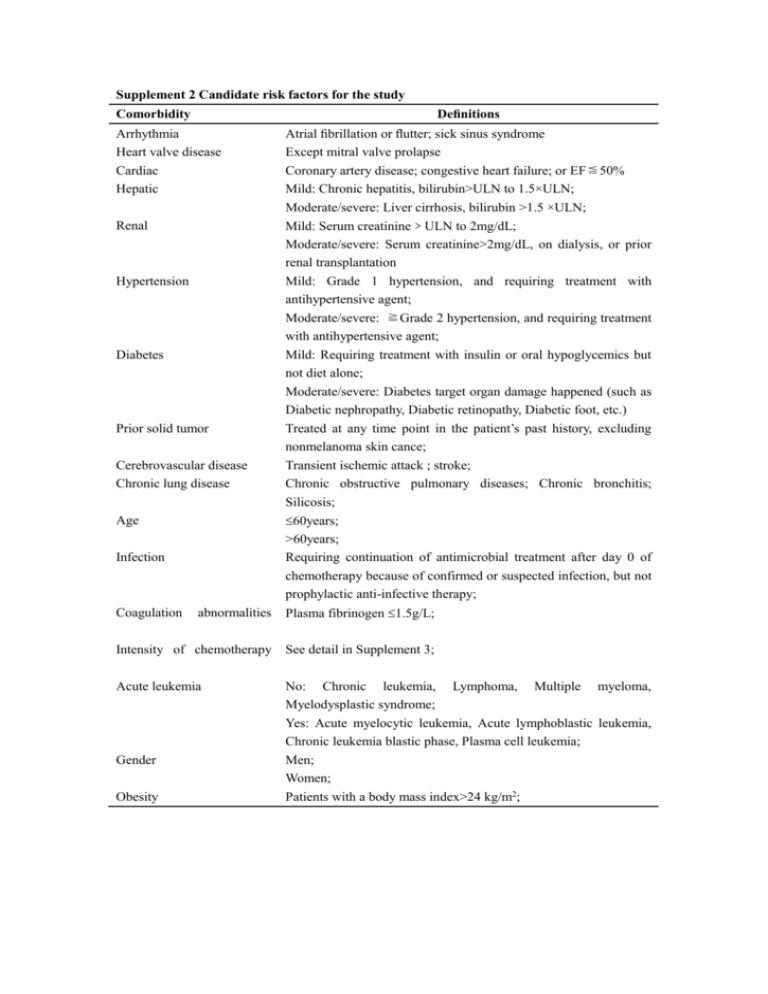
Supplement 2 Candidate risk factors for the study Definitions Comorbidity Arrhythmia Heart valve disease Cardiac Hepatic Renal Hypertension Diabetes Prior solid tumor Cerebrovascular disease Chronic lung disease Atrial fibrillation or flutter; sick sinus syndrome Except mitral valve prolapse Coronary artery disease; congestive heart failure; or EF≦50% Mild: Chronic hepatitis, bilirubin>ULN to 1.5×ULN; Moderate/severe: Liver cirrhosis, bilirubin >1.5 ×ULN; Mild: Serum creatinine﹥ULN to 2mg/dL; Moderate/severe: Serum creatinine>2mg/dL, on dialysis, or prior renal transplantation Mild: Grade 1 hypertension, and requiring treatment with antihypertensive agent; Moderate/severe: ≧Grade 2 hypertension, and requiring treatment with antihypertensive agent; Mild: Requiring treatment with insulin or oral hypoglycemics but not diet alone; Moderate/severe: Diabetes target organ damage happened (such as Diabetic nephropathy, Diabetic retinopathy, Diabetic foot, etc.) Treated at any time point in the patient’s past history, excluding nonmelanoma skin cance; Transient ischemic attack ; stroke; Chronic obstructive pulmonary diseases; Chronic bronchitis; Silicosis; 60years; >60years; Requiring continuation of antimicrobial treatment after day 0 of chemotherapy because of confirmed or suspected infection, but not prophylactic anti-infective therapy; Age Infection abnormalities Plasma fibrinogen 1.5g/L; Intensity of chemotherapy See detail in Supplement 3; Acute leukemia No: Chronic leukemia, Lymphoma, Multiple myeloma, Myelodysplastic syndrome; Yes: Acute myelocytic leukemia, Acute lymphoblastic leukemia, Chronic leukemia blastic phase, Plasma cell leukemia; Men; Women; Patients with a body mass index>24 kg/m2; Coagulation Gender Obesity