Lab 10: Geochemistry of the West Camp Extraction Well
advertisement
EPSC 549
Hydrogeology
Winter, 2005
Lab 9: Geochemistry of the West Camp Extraction Well
Background: The West Camp Extraction Well is located near the bottom of Missoula
Gulch. It pumps groundwater out of the flooded “West Camp” mine workings on Butte
Hill. Table 1 gives a complete chemical analysis of West Camp water, collected in
September of 2000.
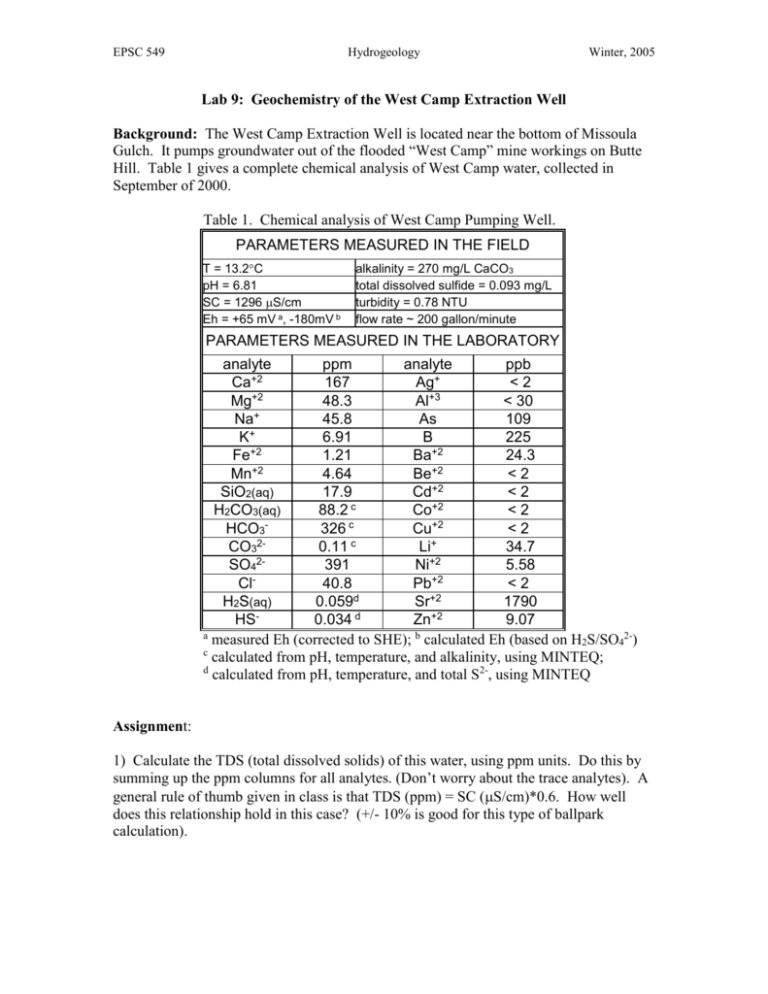
Table 1. Chemical analysis of West Camp Pumping Well.
PARAMETERS MEASURED IN THE FIELD
T = 13.2C
pH = 6.81
SC = 1296 S/cm
Eh = +65 mV a, -180mV b
alkalinity = 270 mg/L CaCO3
total dissolved sulfide = 0.093 mg/L
turbidity = 0.78 NTU
flow rate ~ 200 gallon/minute
PARAMETERS MEASURED IN THE LABORATORY
analyte
ppm
analyte
ppb
+2
+
Ca
167
Ag
<2
Mg+2
48.3
Al+3
< 30
Na+
45.8
As
109
+
K
6.91
B
225
Fe+2
1.21
Ba+2
24.3
+2
+2
Mn
4.64
Be
<2
+2
SiO2(aq)
17.9
Cd
<2
H2CO3(aq)
88.2 c
Co+2
<2
c
+2
HCO3
326
Cu
<2
CO320.11 c
Li+
34.7
SO42391
Ni+2
5.58
+2
Cl
40.8
Pb
<2
H2S(aq)
0.059d
Sr+2
1790
HS0.034 d
Zn+2
9.07
a
b
measured Eh (corrected to SHE); calculated Eh (based on H2S/SO42-)
c
calculated from pH, temperature, and alkalinity, using MINTEQ;
d
calculated from pH, temperature, and total S2-, using MINTEQ
Assignment:
1) Calculate the TDS (total dissolved solids) of this water, using ppm units. Do this by
summing up the ppm columns for all analytes. (Don’t worry about the trace analytes). A
general rule of thumb given in class is that TDS (ppm) = SC (S/cm)*0.6. How well
does this relationship hold in this case? (+/- 10% is good for this type of ballpark
calculation).
EPSC 549
Hydrogeology
Winter, 2005
2) Create a new column that lists each analyte and its concentration in millimolal units.
To convert from ppm to mmol, divide each analyte by its GFW. To convert from ppb to
mmol, divide by (1,000*GFW).
3) Create a new column that lists the milli-equivalents (meq) of charge for each analyte.
To do this, multiply your mmol concentration by the charge of the ion. (+3, -2, etc..).
Keep track of positive or negative charges.
4) Sum up all of the positive charges (cations, in meq), and compare to the negative
charges (anions, in meq). Perform a charge balance calculation, using the following
equation:
charge imbalance (%) =
sumcations sumanions
0.5 sumcations sumanions
x 100
Note that the brackets, denote absolute value. A good analysis should have a charge
imbalance less than 10%. Is this true in this case?
5) Another general rule of thumb is that the measured specific conductance is roughly
equal to 100 x ( meq cations ). How well does this approximation hold for the present
case? Compare to your answer for #1.
6) Calculate the ionic strength of this water. You can do this fairly quickly using your
spreadsheet. Just calculate miZi2 for each ion, sum them up, and divide by 2.
7) Use your answer for #5 and the extended Debye-Huckel equation to calculate
individual ion activity coefficients for Mn2+, Ca2+, SO42- (sulfate ion), and CO32(carbonate ion). Assume T = 15C. (See p. 351-353 of Fetter).
8) Calculate ion activities for Mn2+, Ca2+, SO42-, and CO32-.
Recall that ai = mi x i
9) Calculate the ion activity product (IAP) and saturation index (SI) of this water with
respect to the minerals rhodochrosite (MnCO3), calcite (CaCO3), and gypsum (hydrous
CaSO4). Use the following equation:
SI = log (IAP/Ksp)
Where IAP is the observed “ion activity product”, and Ksp is the experimentallydetermined solubility product for each mineral. Assume that the solubility product (Ksp)
of rhodochrosite is 10–10.39, of calcite is 10–8.48, and of gypsum is 10-4.58. Is this water
super-saturated, under-saturated, or close to equilibrium with these 3 minerals? (“Close
to equilibrium” means that that the saturation index is within +/- 0.3 log units of zero).
10) Calculate the partial pressure of CO2 for West Camp water. Use the following
relationship:
EPSC 549
Hydrogeology
Winter, 2005
KCO2 = mH2CO3/PCO2
where PCO2 is the carbon dioxide partial pressure, in bars. Use data in Table 9.4 to get
KCO2 at 15C. Is your calculated PCO2 of the groundwater less than or greater than the
partial pressure of CO2 in the Earth’s atmosphere? (roughly 0.0002 bars). EXTRA
CREDIT (0.5 pts.): If the sample of West Camp water is left in a bucket and allowed to
equilibrate with air, will the pH of the water go up or down with time? Briefly explain
your answer.
11) Calculate the “total hardness” of this water. From Drever (p. 13):
hardness (in mg/L CaCO3 eq.) = 2.5(ppm Ca2+) + 4.1(ppm Mg2+)
Classify this water as “soft”, “moderately hard”, “hard”, or “very hard”:
Hardness range (mg/L CaCO3)
0-60
61-120
121-180
More than 180
Classification (Hem, p. 159)
Soft
Moderately hard
Hard
Very hard
12). For certain divalent metals, the Montana State WQB-7 standard for aquatic life is
referenced to total hardness. In the long version of WQB-7, you will find the following
information.
Chronic regulatory standard (ppb) = exp{mc[ln(hardness)]+bc}
Zn2+
Aquatic life, chronic exposure
mc
bc
0.8473
0.884
Use this equation to calculate the regulatory standard for chronic exposure of aquatic life
to Zn, for the actual hardness of MSD water. [NOTE: the maximum hardness that WQB7 will allow you to input is 400 mg/kg. If your calculated hardness is greater than this,
then enter 400 mg/kg). Does this water exceed the State standard?
13) The new EPA arsenic standard for human health is 10 ppb. Does West Camp water
exceed this standard? How many grams of As are pumped out of the West Camp flooded
mine workings in 1 day?






