Biogeo Cycles Part 1
advertisement

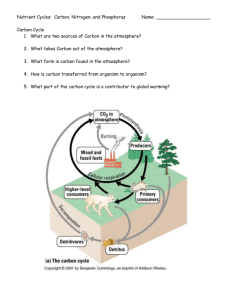
The Carbon Cycle: Summary of Cycle In the Carbon cycle, carbon moves everywhere! Carbon is taken from the atmosphere in the form of CO2 and through photosynthesis produces glucose with the help of the Sun for the plants to use. The carbon atoms are then transferred into animals as the plants are eaten. Living animals release carbon back into the atmosphere by releasing carbon dioxide in the process of respiration and eventually when the animals die, their bodies as well as other detritus decay and put carbon into the ground where the carbon atoms can become fossil fuels in millions of years. These burning of these fossil fuels by humans release the carbon into the atmosphere as well. Though the oceans release carbon into the atmosphere, it also absorbs some of the carbon from the atmosphere and the cycle repeats itself over and over again as shown in this diagram. Multiple Choice Questions o What is the purpose of a carbon sink? (e) a) b) c) d) e) o Release carbon into the air Absorb CO2 from the atmosphere Create deposits for fossil fuels Keep CO2 from accumulating at amore rapid rate in the atmosphere Both b and d Which option is not a description of part of the carbon cycle? (a) a) Plants take CO2 from the soil where organic carbon in manure and fertilizer is used to sustain soil using plants. b) Animals gather the carbon atoms from eating plants and release it through respiration c) As dead animals and other detritus such as leaves or wood decays, the carbon atoms enter the ground d) The carbon atoms found in the ground become fossil fuels that is potentially used by human, therefore releasing carbon back into the atmosphere e) The ocean release and absorbs some of the carbon into or from the atmosphere. As animals release carbon in the form of carbon dioxide through respiration, the producers or other primary producers take up carbon dioxide through photosynthesis. More specifically, the carbon atoms from CO2 that are taken from the atmosphere become carbon atoms of organic molecules through photosynthesis that eventually go into the food webs and become part of the ecosystem. Through respiration, the organic carbon atoms are released into the atmosphere. For the ocean ecosystem however, phytoplankton and macroalgae removes CO2 from the huge pool of inorganic carbonates in seawater through photosynthesis, and feeding moves the organic compounds through marine food webs. And the carbon is returned to inorganic carbonates in the solution due to the respiration of the biota. Photosynthesis Equation: 6CO2 + 6H2O + Energy C6H12O6 + 6O2 Cellular Respiration Formula: C6H12O6 + 6O2 6CO2 + 6H2O + Energy B. Carbon is released into the atmosphere by the respiration of organisms, the burning of fossil fuel, deforestation/ forest fires, and the release of carbon from the ocean. C. CO2 is 0.03 percent of the atmosphere. Today we are at 387 parts per million of CO2. The CO2 was at 298 parts per million in 1900. D.Four carbon sinks are the oceans, terrestrial ecosystems, fossil fuel deposits, and the atmosphere. Phosphorus Cycle Summary: Phosphorus is one of the most important elements to living organisms. It is found in DNA, phospholipids, and other majorly important molecules. It occurs in nature as phosphate in ocean sediments and rocks. Weathering breaks it down into the soil so that the plants are able to absorb the phosphates from the soil. Animals eat the plants, and then when they die, the decomposers break down the tissue of the dead animals allowing the phosphorus to be reincorporated into the soil. Ocean runoffs allow the phosphorus to enter the ocean where it eventually sinks to the bottom to form new rocks. In which of the following is phosphorus not important? A) DNA B) Phospholipids C) ATP D) RNA E) H20 How are animals able to get phosphorus? A) They eat plants which have absorbed the phosphorus from the soil. B) They eat the rocks which contain phosphorus. C) They are born with all the phosphorus they will need. D) They produce it themselves. E) They get it from the sun. Question A: The phosphorus cycle is different from the other cycles because it does not include a gas phase. Question B: Mining, Fertilizing, Sewage Runoff can contribute to extra phosphorus which will help some species like Algae take over. Question C: Fertilizers contain an abundant amount of phosphorus. Algae has a growth advantage when there is a large amount of phosphorus contributing to its bloom problems since a large amount of Algae limits the amount of other species that can survive. Nitrogen Cycle Summary: Nitrogen is the most abundant elements on our planet; however the majority of the earth’s life can not use nitrogen in its original form. Nitrogen is also a requirement for life, as it exists in amino acids and various other essential aspects of life. In order to use it nitrogen needs to be fixed into plants. This process, aptly named nitrogen fixation, involves a free living enzyme called Nitrogenase which converts the nitrogen to ammonia. This usually occurs is the legume family of plants. The fixing of nitrogen also can occur during lightning strikes. Humans also have the ability to create ammonia and use it as a fertilizer. The decay of dead animals also adds nitrogen to the soil through a process known as ammonification in which the nitrogen in the animal is converted to ammonium by decomposers.. However, plants still can't use pure ammonium and the nitrogen must go through another step before becoming usable. Nitrification is the process where bacteria convert the ammonium to nitrates which is then used by the plants through the roots. In order to ensure that the nitrogen doesn't just build up in the ground there is a process called denitrification, where bacteria in the soil convert the nitrates to nitrogen gas and put it back into our atmosphere. 1. In what family of plants is nitrogen usually fixed. a) Coniferous Trees b) Sea Grass c) Wheat d) legumes e) Deciduous Trees 2. Name the state where to many dissolved nutrients are in a body of water, such as when excesses of human made ammonia run off into the seas causing algal blooms. a) Hyperautophagy b) eutrophication c) Disequitable overload d) Red algal blooms e) Transhydrodiction Question A In the roots of legumes there live many bacteria who through their own metabolic processes are able to convert the nitrogen gas in to ammonia or ammonium, depending of the acidity of the soil. Converting nitrogen gas into a more usable form is extremely difficult and is the reason that this process is so specialized. While ammonium may be usable by a few plants, the majority cannot use it and another process is required. Nitrifying bacteria oxidize the ammonia creating nitrite then nitrate allowing for the nitrogen to be assimilated into the plants. Question B Ammonification is the process by which nitrogen stored in organic matter such as animals and plants is converted into ammonium. Decomposers in the soil recycle the nitrogen, creating ammonium which allows for the nitrogen to enter the process of nitrification. Nitrification is the conversion of the ammonium to nitrites then nitrates by aerobic bacteria. These nitrates are usable by plants and are assimilated into the plants system. The nitrates are used for creating proteins as well as amino acids within the plants. Animals can only assimilate nitrogen through the consumption of the plants or of other animals. Not all of the nitrates are assimilated and some enter a process known as denitrification. Some bacteria use the nitrates for oxygen for metabolism leaving the nitrogen to be freed and return to the atmosphere. Sulfur Cycle SO4 Formation The weathering of rocks or the eruption of a volcano opens the sulfur to the air allowing the sulfur to bind with oxygen and create SO4. Common source Erosion of rocks and eruption of volcanoes puts this gas into the air and rain brings it down to the plants and microorganisms. Ecological importance Sulfate is converted into organic forms by microorganisms and plants to move sulfur through the food chain since sulfur is an important element in amino acids in living organisms. SO2 Formation 90% of Sulfur dioxide released into the air is a result of human action when we burn coal, crude oil or other fuels. Common source Fossil fuel combustion, industrial processes and electricity generation Ecological importance Sulfur dioxide dissolves in water to form an acid which is harmful, especially in the form of acid rain which can spread to many distance locations. Humans are adding too much sulfur to the air SOx so the ecological impact is a negative one. CH3 SCH3 Formation Phytoplankton and bacteria produce this byproduct to the air. Common source Oceans and bacterial metabolism Ecological importance In terms of the sulfur cycle, this compound is one of the most abundant organic sulfur molecules in the air. H2SO4 Formation Sulfuric acid forms when SOx dissolves in water, and over thirty million tons of sulfuric acid are produced in the US every year. Common source Acid Rain and man Ecological importance Sulfuric acid is an important compound in many industries, but it is the main constituent of acid rain. H2 S Formation Bacteria produces this gas from their metabolism from the unusable sulfates in the ground so that it can be put into the atmosphere where it later is rained on plants. Common source The most common source is metabolism of bacteria. Ecological importance This is extremely important because plants are unable to use the sulfate in the ground unless bacteria are able to convert it into a more useable form. The Water Cycle During the water cycle the water will rise high into the atmosphere cooling the warm water vapor down. This vapor turns into liquid as heat leaves it in the process known as condensation. This new liquid in the atmosphere becomes too heavy for the atmosphere and falls to earth as rain, snow, sleet, or hail, this is known as precipitation. Once the precipitation lands on the ground some of the water will seep into the soil joining earths ground water in a process known as infiltration. The water that does not seep into the ground because of varying ground conditions becomes runoff, or water that flows across the land into the rivers and oceans. In order for water to return to the atmosphere evapotranspiration has to occur. This is a combination of two process known as evaporation and transpiration. Evaporation is water heating up on the earth, turning into water vapor and rising into the atmosphere. Transpiration occurs when plants take up water through their roots, use it and release it through their leaves as water vapor. Humans have the ability to alter these cycles through things like building dams to prevent runoff, releasing pollution that combines with the water vapor to create acid rain, and adding to many fertilizers to ground water for agriculture. In order to ensure clean healthy water for all generations we must make preserving the water cycle one of our utmost priorities.