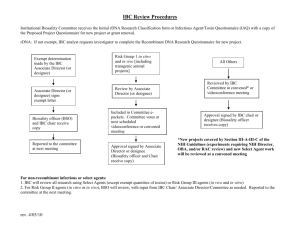
SubForm: Exogenous Substances, Pathogens, Chemical
advertisement

SubForm: Exogenous Substances, Pathogens, Chemical/Carcinogens, Volatile Gases, Recombinant DNA (IBC), Biohazardous (IBC), and Radioactive Materials. For tumor studies and ascites production, please use SubForm: Tumors in Animals instead. Use a separate Attachment for each recipient species 1. Recipient species: 2. Identify the substance and its source. Describe whether the substance is biological and give its source (species, preparation, synthesis, purification, etc as appropriate). If the substance is a proprietary compound or agent that cannot be explicitly described, address the issues listed in item 3 (Proprietary Substances). If the substance is of biological origin, state what steps have been taken to render it free of adventitious infectious agents to man and the recipient species or provide documentation that the substance has been tested (via PCR, MAP test, RAP test, etc.)? Animals given biologic material with an unknown infectious potential will be maintained in quarantine for the duration of the study. 3. Proprietary Substances: a) Provide written assurance that the substance, at the levels/doses to be used in the animal protocols, is not toxic or otherwise harmful to animals/humans involved in the proposed research. This assurance should briefly describe how the substance has been subjected to standard in vitro tests for toxicity or mutagenicity. b) If evaluation of in vivo toxicity or potential harmfulness of the proprietary substance is the goal of the proposed animal experimentation, indicate how the protocol will estimate the maximum tolerated dose (MTD) and no effect dose level (NOEL) for that substance in the animal subjects.] 4. Administration. Animal Biosafety Level: You can reference the ABSL Hazard levels at: ARC Training Modules Dosage: Volume: Route: Frequency Maintenance Duration: Animals will be anesthetized or sedated for administration Yes No If Yes, complete SubForm: Anesthesia, Analgesia Or Paralysis 5. What are the experimental endpoints and criteria for interventional euthanasia? Biohazards and/or Recombinant DNA: If the project requires approval for any of the agents below, a copy of the approved safety form from the appropriate safety committee must be provided before activation of the protocol, with the exception of transgenic and knockout mice, as work can proceed on these while the application is pending. Investigators using hazardous chemicals and/or radioactive materials must not dispose of their animals without contacting the Department of Occupational and Environmental Safety for instructions at 216-368-2906 or 216-368-2907. Mixed waste (infected animals labeled with radioactive material or infectious and radioactive waste) cannot be submitted for disposal. Submit this page to D.O.E.S. with each submitted safety form. 6. Identify the agent in the appropriate category. (Pathogen) Infectious agents (Pathogen). Identify: IACUC_Pathogen_Form.doc Hazardous chemicals, including chemical carcinogens (Carcinogen) Identify: For forms, contact Marc Rubin 368-2907 and download IACUC_Chemical_Form.doc Volatile Anesthetics Identify examples: isoflurane, ether, halothane, ethyl carbamate, nitrous oxide, methoxyflurane and Location of Use. Recombinant DNA (IBC) Describe: Production of transgenic and knockout mice needs to be approved for Recombinant DNA, in accord with NIH policy. For forms, Institutional Biosafety Committee (IBC).cfm Importation of Bio-hazardous Animals (Non-Standard Vendor) Describe: The importation and exportation of animals which present a biohazard must be approved by the CWRU IBC. For forms, see nonstandard_vendor_import.pdf nonstandard_vendor_export.pdf Radioactive Materials. Describe: For forms, RadSafety/application.pdf 7. Specify the containment methods to be followed in protecting other research animals and personnel from any of the agents listed above. 8. Describe the procedures required for the safe handling and disposal of contaminated animals, caging, bedding, food and materials associated with this study. Describe methods for removal of radioactive waste and monitoring of radioactivity, if applicable. 9. Describe scavenging systems used to capture waste anesthetic gases if volatile anesthetics are used. The importation of animals which present a biohazard must be approved by the CWRU IBC. For forms http://ora.ra.cwru.edu/research/orc/rdna/indxPg_Cwru_ibc.cfm This protocol will not be approved by IACUC without appropriate Committee and D.O.E.S. approvals. Pathogen Safety Officer Name (Chair or Designee) Print or type: Signature Date: Radiation Safety Officer Name (Chair or Designee) Print or type: Signature Date: Chemical Carcinogen/Regulated Chemicals Officer Name (Chair or Designee) Print or type: Signature Date: D.O.E.S. Verification of Compliance Printed Name: Position: Signature: Date: