Yeast Fermentation Lab: Glucose & Gas Production
advertisement

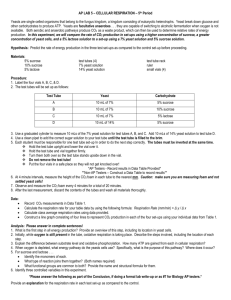
Investigation: Yeast fermentation (Anaerobic Cellular Respiration) Name: _______________________________☺ Purpose: Determine the effects of glucose concentration on yeast fermentation by measuring the amount of gas produced. Materials: 4 small test tubes 4 large test tubes test tube rack yeast solution (8g/L) markers distilled water graduated cylinder (25 mL) glucose solutions (6%, 3%, 1%) masking tape metric ruler Procedure: 1. Label the small test tubes as control (distilled water), 1%, 3% and 6%. 2. Repeat step 1 with the large test tubes. 3. Measure out 12 mL of the 6% glucose solution and place it in the corresponding small test tube. 4. Repeat step 3 with the 3% and 1% solutions. 5. Measure out 12 mL of distilled water and pour it in the small test tube labeled control. 6. Top up each of the four small test tubes with yeast solution. 7. Place a large test tube over each small test tube, invert and place in the test tube rack. 8. Label the test tube rack with your names. 9. After 24 hours, measure and record the amount of gas in each test tube in centimeters using a ruler. 10. Smell (but do not taste!) the contents of the large test tubes. Record your observations. Investigation: Yeast fermentation Name: _____________________ Due date: ______________________ Assessment: /15 Each student will submit an informal lab report for this investigation which includes: o Class handout with Title, Purpose, Materials, Procedure o Observations – qualitative (burning splint result) and quantitative (gas volumes) o Answers to the Discussion questions below (using complete sentences) Observations: include a proper data table with your quantitative observations as well as your qualitative observations in your observations section. (5 marks) Discussion: 1. a) Describe the process of anaerobic respiration in yeast. (3 marks) b) Yeast cells can undergo aerobic respiration (using oxygen) as well as anaerobic respiration (without the use of oxygen). How was the experiment set up to ensure that only anaerobic respiration was happening? (1 mark) 2. a) Based on the burning splint test, what gas was most likely produced? (1 mark) b) Many other gases have the same result for the burning splint test. To confirm the identity of the gas, a limewater test is often done. Describe this test and the positive result for this particular gas. (2 marks) 3. a) Identify the independent variable and the dependent variable for this investigation. (1 mark) b) Write a conclusion for the experiment in terms of these two variables. (1 mark) c) After a period of time, the production of gas in the 6% glucose solution will stop even with glucose remaining. Suggest a plausible reason for this. (1 mark)