CRACKING GEL PROCEDURE
advertisement

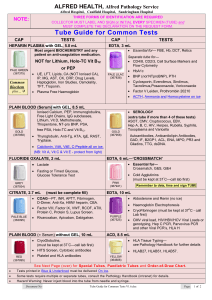
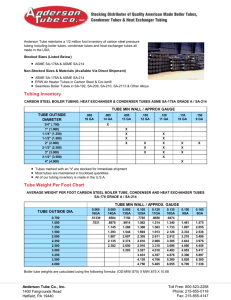
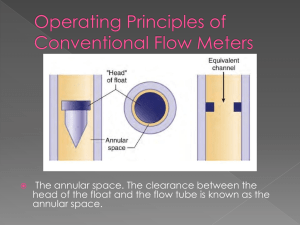
Jenny Gipson 10-16-2001 CRACKING GEL PROCEDURE 1. Get out the appropriate number of sterile, 1.5ml microfuge tubes. 2. Add 30µl of cracking gel buffer to each tube. 3. Pick a colony with a toothpick and twirl the toothpick vigorously in the tube with the cracking buffer. Repeat for each colony. If your colonies are small, you may need to streak them on a gridded plate to grow up overnight. 4. You will want to prepare a sample of supercoiled pUC to run against your samples. 5. Close the tubes. 6. Leaving them in a microfuge tube rack, put in an incubator at 37ºC, 225rpm for 15min. You may want to tape paper towels over the tubes so that they won't come out of the rack. Also make sure to secure the rack well in the shaker. 7. Prepare a .8% agarose (ethidium bromide) gel with enough wells to run the samples. 8. Spin the tubes (at 4C or room temp.) for 15 minutes at 15,000rpm. 9. Before loading each sample, remove the goo ball at the bottom of tube by scraping your pipet tip from the bottom of the tube up the side of the tube. 10. Then load the sample into the well. 11. Be sure and load ladders. 12. Run the gel at 80-100volts for 1-11/2 hrs (or long enough to see placement). 13. Look at under UV and take picture.