Salame - The City College of New York
advertisement

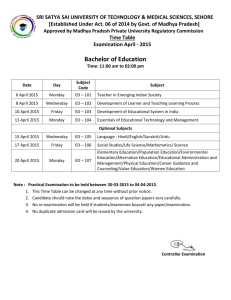
The City College of New York Department of Chemistry Chemistry 243: Quantitative Analysis Professor Issa Salame Class Time and Days: 11:00 am – 12:15 pm, Mondays and Wednesdays Class Location: MR 1307 Telephone: (212) 650 – 6924 Office Location: MR 1224 Email address: Prof.salame@gmail.com ************************************************************************ Catalog Description: Volumetric, Spectrophotometric, Electrochemical and Chromatographic Analyses Prerequisites: Chem 10401 Co-requisites: Hours/Credits: 7 hours per week, 3 LECT., 4 LAB - 4 credits Textbook: Analytical Chemistry: An Introduction, 7th ed. by D.A. Skoog, D. M. West, F. J. Holler and S.R. Crouch (1999) General Objective: This course intends to introduce the bases of analytical chemistry for chemistry and biochemistry majors. The emphasis is put either on understanding the theoretical aspects of quantitative analysis or problem solving skills. LEARNING GOALS: Students should: Know the physical bases for analytical methods discussed during the course and understand them Be aware of the sources of errors and have knowledge how to avoid them Know how to calculate the amount of analyte in the specific application of each method Know how to obtain calibration curve and how to use it for an analytical purpose Know the criteria, which are used for choosing the methods for a particular analysis. CONCEPTUAL THINKING OBJECTIVES: Reading: cause-effect logic, hypothesis testing, summarizing logic Writing: cause-effect links, objective designing, experiment planning Data analysis: relevant data sources, data treatment, qualitative and quantitative evaluation, data consistency, error analysis Models: cause-effect, correlation, trends LEARNING ACTIVITIES: Text reading Class‐time (lecture) Hand on experience (laboratory) 1 Group discussion Computer‐aid instruction Problem solving (individual) Student‐instructor consulting STUDENT LEARNING OUTCOMES The objectives of this course contribute to the following departmental educational outcomes: 1. Define the physical and chemical principles of volumetric, gravimetric, electrochemical and basic spectroscopic methods (AA, AE, FTIR). 2. Identify which analytical method should be used to quantitatively determine certain level of a target analyte in various matrices. 3. Define the principles and goals of analytical separations including chromatographic techniques ( GC, HPLC), 4. Understand multiple equilibria in solutions and effectively use chemical equilibrium toward determination of the target analyte conc. 5. Understand the significance of the random and systematic errors, know the ways to minimize/avoid them, and use the basic statistical evaluation of errors (standard deviation, variation, t-test, Qtest). 6. Understand and apply the purpose, principle and significance of calibration techniques for quantitative determination of analyte. 7. Describe in terms of chemical reactions and equilibrium constants all steps used to quantitatively determine the concentration of analyte 8. Successfully perform volumetric, gravimetric, spectroscopic and chromatographic determination of the target analyte concentration and evaluate the experimental error. 9. Write a laboratory report including data and error analysis. Department outcomes a a, j a a, e a, c, d, e a a, e a, b, c, i g, i, k Assessment tools: The final grade is calculated as follows: Best three scores of the four in-class examinations (35%) Quizzes (10%) Final Exam (30%) Laboratory (25%) * The lowest grade exam will be dropped. Missing an exam will result in receiving a zero grade for that particular exam and thus dropping that grade. There will not be any Make-up Exams. Office Hours: Mondays and Wednesday 1:00 pm – 3:00 pm Or by appointment Office is located in the science building in room MR 1224 Absence Policy (Attendance) Students are expected to attend every class session of each course in which they are enrolled and to be on time. An instructor has the right to drop a student from a course for excessive absence. Students are advised to determine the instructor’s policy at the first class session. They should 2 note that an instructor may treat lateness as equivalent to absence. (No distinction is made between excused and unexcused absences.) Each instructor retains the right to establish his or her own policy, but students should be guided by the following general College policy: In courses designated as clinical, performance, laboratory or field work courses, the limit on absences is established by the individual instructor. For all other courses, the number of hours absent may not exceed twice the number of contact hours the course meets per week. When a student is dropped for excessive absence, the Registrar will enter the grade of WU. Statement on Academic Integrity The CCNY policy on academic integrity will be followed in this course. The document can be found through the CCNY website by clicking on Current Students Academic Services Policy on Academic Integrity. All students must read the details regarding plagiarism and cheating in order to be familiar with the rules of the college. Cases where academic integrity is compromised will be prosecuted according to these rules. In addition, the Policy of Academic Integrity can be found in the Undergraduate Bulletin 2007-2009 in Appendix B.3 on page 312. Disability In compliance with CCNY policy and equal access laws, appropriate academic accommodations are offered for students with disabilities. Students must first register with The AccessAbility Center for reasonable academic accommodations. The AccessAbility Center is located in the North Academic Center, Rm. 1/218. Tel: (212) 650-5913. Under The Americans with Disability Act, an individual with a disability is a person who has a physical or mental impairment that substantially limits one or more major life activities. If you have any such issues, I encourage you to visit the AccessAbility Center to determine which services may be appropriate for you. Courtesy Noise and excessive chatter, eating, drinking, or use of unauthorized electronic equipment is not allowed in the classroom. Make-up examination for INC grades INC may be assigned to students who have a passing grade (average on all the exams) in the course but who are unable to take the final examination due to conflict with another scheduled examination, death of spouse, injury sustained in a catastrophic incident, etc. (proof is also required). An Incomplete Grade Agreement form must be signed by the Instructor before the student is allowed to take the makeup exam. Payment of a fee at the Bursar's office is required in order to take the makeup examination. Makeup exam for INC grades in Chemistry courses will be completed no later than two weeks after the end of classes. DATE CHAPTERS and High priority areas Class Schedule August 27th Monday Chapter 5. Errors in Chemical Analysis Chapter 6. Random Errors in Chemical Analysis August 29th Wednesday Chapter 7. Application of Statistics to Data Treatment September 3rd Monday *** NO CLASSES *** College Closed *** Labor Day 3 September 5th Wednesday Chapter 11. Titrimetric Methods of Analysis September 10th Monday Chapter 4. Aqueous Solution Chemistry September 12th Wednesday Chapter 9. Effect of Electrolyte on Chemical Equilibria September 17th Monday *** NO CLASSES *** College Open *** September 19th Wednesday Chapter 10. Application of Equilibrium Calculations to Complex Systems September 24th Monday Catch-Up and Review for first examination September 26st Wednesday *** NO CLASSES *** College Open *** October 1st Monday *** FIRST EXAMINTION *** October 3rd Wednesday Chapter 12. Theory of Neutralization Titration October 8th Monday October 10th Wednesday *** NO CLASSES *** College Closed *** Columbus Day Monday Schedule Chapter 13. Titration Curves for Complex Acid/Base Systems October 15th Monday Chapter 13. Titration Curves for Complex Acid/Base Systems October 17th Wednesday Chapter 14. Application of Neutralization Titrations October 22nd Monday Chapter 15. precipitation Titrimetry: Complex Formation Titration October 24th Wednesday Catch-up and Review for Second Examination October 29th Monday *** SECOND EXAMINTION *** 4 October 31st Wednesday Chapter 16. Introduction to Electrochemistry November 5th Monday Chapter 17. Application of Standard Electrode potential November 7th Wednesday Chapter 18. Application of Oxidation Reduction Titration November 12th Monday Chapter 19. Potentiometry November 14th Wednesday Chapter 20. Other Electroanalytical Methods November 19th Monday *** THIRD EXAMINATION *** November 21st Wednesday Chapter 21. Introduction to Spectrochemical Methods Chapter 23. Molecular Absorption Spectroscopy November 26th Monday Chapter 23. IR and Atomic Spectroscopy November 28th Wednesday Chapter 24. Analytical Separations December 3rd Monday Chapter 24F. An Introduction to Chromatographic Methods December 5th Wednesday Chapter 25A. Gas Liquid Chromatography Chapter 25 BC. High Performance Liquid Chromatography Chapter 26 A. High Performance Liquid Chromatography December 10th Monday *** FOURTH EXAMINATION *** December 12th Wednesday Summary of Analytical Methods Final Examination will be scheduled during the final exam period between Thursday the 14th and Thursday the 21st of December of 2012. Our scheduled final examination date is the 19th of December from 10:30 am until 12:45 pm. Study Guides: 1. Plan at least three hours of study (reading the chapter and completing the problem sets “homework”) time for every hour you spend in class. 2. Do the problem sets individually (without help from friends or classmates) initially. Please look at a related problem in the solution manual to help you solve the assigned 5 problem. If you are still unable to solve the problem, then ask a friend, classmate, workshop leader, TA, or Professor for help. 3. Read the book and take notes as you read. 4. Seek help when you have difficulty (office hours, tutoring, study groups). “I hear, I forget. I see, I remember. I do, I understand.” Chinese proverb 6