ARTICLES RELATED TO Conjugated system
advertisement
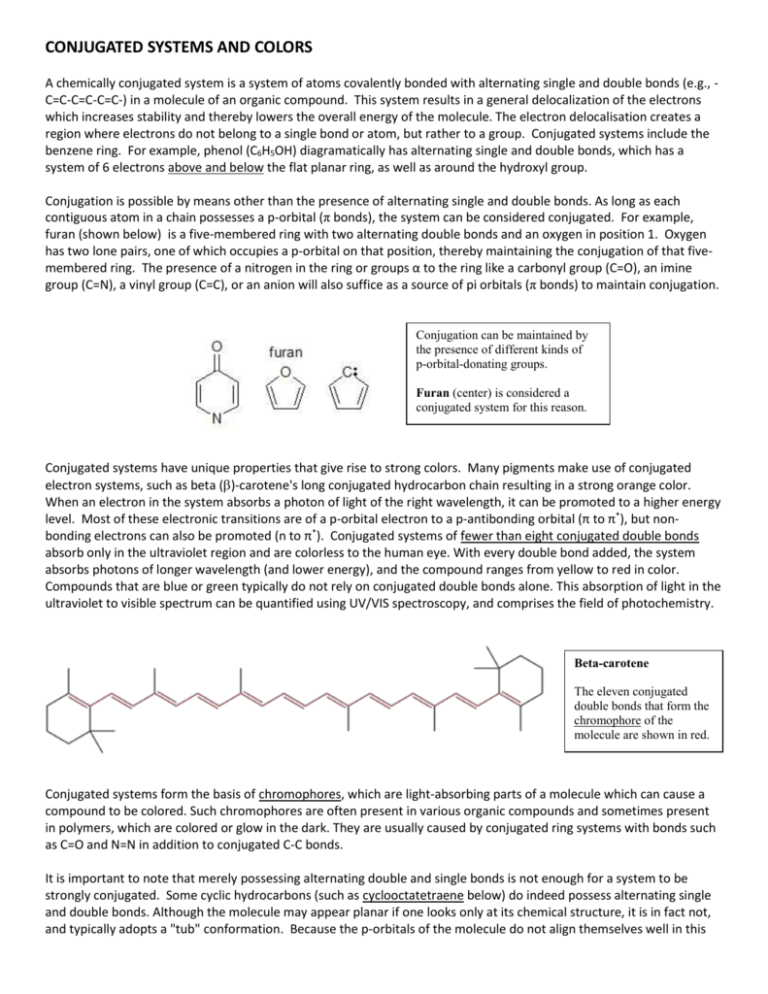
CONJUGATED SYSTEMS AND COLORS A chemically conjugated system is a system of atoms covalently bonded with alternating single and double bonds (e.g., C=C-C=C-C=C-) in a molecule of an organic compound. This system results in a general delocalization of the electrons which increases stability and thereby lowers the overall energy of the molecule. The electron delocalisation creates a region where electrons do not belong to a single bond or atom, but rather to a group. Conjugated systems include the benzene ring. For example, phenol (C6H5OH) diagramatically has alternating single and double bonds, which has a system of 6 electrons above and below the flat planar ring, as well as around the hydroxyl group. Conjugation is possible by means other than the presence of alternating single and double bonds. As long as each contiguous atom in a chain possesses a p-orbital (π bonds), the system can be considered conjugated. For example, furan (shown below) is a five-membered ring with two alternating double bonds and an oxygen in position 1. Oxygen has two lone pairs, one of which occupies a p-orbital on that position, thereby maintaining the conjugation of that fivemembered ring. The presence of a nitrogen in the ring or groups α to the ring like a carbonyl group (C=O), an imine group (C=N), a vinyl group (C=C), or an anion will also suffice as a source of pi orbitals (π bonds) to maintain conjugation. Conjugation can be maintained by the presence of different kinds of p-orbital-donating groups. Furan (center) is considered a conjugated system for this reason. Conjugated systems have unique properties that give rise to strong colors. Many pigments make use of conjugated electron systems, such as beta ()-carotene's long conjugated hydrocarbon chain resulting in a strong orange color. When an electron in the system absorbs a photon of light of the right wavelength, it can be promoted to a higher energy level. Most of these electronic transitions are of a p-orbital electron to a p-antibonding orbital (π to π*), but nonbonding electrons can also be promoted (n to π*). Conjugated systems of fewer than eight conjugated double bonds absorb only in the ultraviolet region and are colorless to the human eye. With every double bond added, the system absorbs photons of longer wavelength (and lower energy), and the compound ranges from yellow to red in color. Compounds that are blue or green typically do not rely on conjugated double bonds alone. This absorption of light in the ultraviolet to visible spectrum can be quantified using UV/VIS spectroscopy, and comprises the field of photochemistry. Beta-carotene The eleven conjugated double bonds that form the chromophore of the molecule are shown in red. Conjugated systems form the basis of chromophores, which are light-absorbing parts of a molecule which can cause a compound to be colored. Such chromophores are often present in various organic compounds and sometimes present in polymers, which are colored or glow in the dark. They are usually caused by conjugated ring systems with bonds such as C=O and N=N in addition to conjugated C-C bonds. It is important to note that merely possessing alternating double and single bonds is not enough for a system to be strongly conjugated. Some cyclic hydrocarbons (such as cyclooctatetraene below) do indeed possess alternating single and double bonds. Although the molecule may appear planar if one looks only at its chemical structure, it is in fact not, and typically adopts a "tub" conformation. Because the p-orbitals of the molecule do not align themselves well in this The native conformation of cyclooctatetraene. Adjacent double bonds are not coplanar, so there is not strong conjugation between them. non-planar molecule, the electrons are not as easily shared between the carbon atoms. They can be still considered conjugated, but they are not considered antiaromatic. Thus, cyclooctatetraene is a 4n system but neither aromatic or antiaromatic because the molecule escapes a planar geometry. Antiaromatic molecules are cyclic systems containing alternating single and double bonds, where the pi electron energy of antiaromatic compounds is higher than that of its open-chain counterpart. Therefore antiaromatic compounds are unstable and highly reactive; often antiaromatic compounds distort themselves out of planarity to resolve this instability. Examples of antiaromatic systems are cyclobutadiene (A), the cyclopentadienyl cation (B) and the cyclopropenyl anion (C). By adding or removing an electron pair via a redox reaction, a π system can become aromatic and therefore more stable than the original non-aromatic (a polyunsaturated cyclic compound that is not aromatic) or anti-aromatic compound, for instance the cyclooctatetraenide dianion. The IUPAC criteria for antiaromaticity are as follows: ● The molecule must have 4n π electrons where n is any integer. ● The molecule must be cyclic. ● The molecule must have a conjugated pi electron system. ● The molecule must be planar. Conjugation in cyclic structures results in aromaticity, an unusual stability found in cyclic conjugated systems such as benzene. Other important conjugated structures include Vitamin A ,Vitamin D, and hemoglobin. So far you have learned that inorganic compounds of transition metals with unfilled d orbitals reflect color, but these are not the only ones to do so. Organic compounds with conjugated systems can also reflect colored light and many of these comprise some of the brightest natural pigments in plants and animals as well as many synthetic dyes. Let’s take a look at the differences in leaf colors. Autumn Leaf Colors The color of a leaf results from an interaction of different pigments produced by the plant. The main pigment classes responsible for leaf color are porphyrins, carotenoids, and flavonoids. The color that we perceive depends on the amount and types of the pigments that are present. Chemical interactions within the plant, particularly in response to acidity (pH) also affect the leaf color. PIGMENT CLASS COMPOUND TYPE COLORS ------------------------------------------------------------------------------------------------------------------Porphyrin chlorophyll green ------------------------------------------------------------------------------------------------------------------Carotenoid carotene orange lycopene red xanthophyll yellow ------------------------------------------------------------------------------------------------------------------Flavonoid flavone yellow flavonol yellow anthocyanin red, blue, purple, magenta -------------------------------------------------------------------------------------------------------------------- Chemistry of Plant Pigments Let's take a closer look at the molecular structures and functions of the three major plant pigment classes: Porphyrins : All porphyrins have the following ring structure. Notice the conjugated system present: The primary porphyrin in leaves is a green pigment called chlorophyll. There are different chemical forms of chlorophyll (e.g., chlorophyll a and chlorophyll b), which are responsible for carbohydrate synthesis within a plant. Chlorophyll is produced in response to sunlight. As the seasons change and the amount of sunlight decreases, less chlorophyll is produced, and the leaves appear less green. Chlorophyll is broken down into simpler compounds at a constant rate, so green leaf color will gradually fade as chlorophyll production slows or stops. Carotenoids: Carotenoids are terpenes made of isoprene subunits. Examples of carotenoids found in leaves include lycopene, which is red, and xanthophyll, which is yellow. Light is not needed in order for a plant to produce carotenoids, therefore these pigments are always present in a living plant. Also, carotenoids decompose very slowly as compared to chlorophyll. Notice the conjugated systems in these pigments. Flavonoids: Flavonoids contain a diphenylpropene subunit. Examples of flavonoids include flavone and flavol, which are yellow, and the anthocyanins, which may be red, blue, or purple, depending on pH (see next page). Anthocyanins, such as cyanidin, provide a natural sunscreen for plants. Because the molecular structure of an anthocyanin includes a sugar, production of this class of pigments is dependent on the availability of carbohydrates within a plant. Anthocyanin color changes with pH, so soil acidity affects leaf color. Anthocyanin production also requires light, so sunny days are needed for the brightest fall colors! Autumn Color Change When leaves appear green, it is because they contain an abundance of chlorophyll. Chlorophyll masks other pigment colors. Anthocyanins, in turn, mask carotenoids. As summer turns to autumn, decreasing light levels cause chlorophyll production to slow. However, the decomposition rate of chlorophyll remains constant, so the green color will fade from the leaves. At the same time, anthocyanin production in leaves increases, in response to surging sugar concentrations. Leaves containing primarily anthocyanins will appear red. Leaves with good amounts of both anthocyanins and carotenoids will appear orange. Leaves with carotenoids but little or no anthocyanins will appear yellow. In the absence of these pigments, other plant chemicals also can affect leaf color. An example includes tannins, which are responsible for the brownish color of some oak leaves.