Principles of Spectroscopy
advertisement

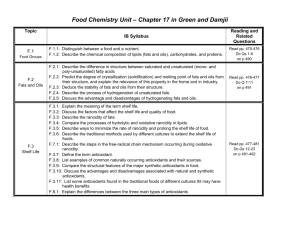
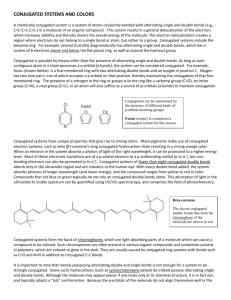
Principles of Spectroscopy Wavelength (nm) 10-4 10-1 10 – 400 (most useful: 200-400) 400-700 Type Visible Gamma Rays X-rays Ultraviolet Causes changes to Nucleus Inner Electrons Pi electrons Visible Valence e- 400 500 600 700 104 Infrared 109 1014 Microwaves Radiowaves Molecular vibrations Rotations Nuclear Spin Energy/Frequency Violet Blue Green Yellow Orange Red En er g y D e cr e as es Fr e q u e n cy D e cr e as es W a v el e n gt h In cr e as es Only certain amounts of energy can be absorbed by molecules, atoms, electrons. Because of this we say energy is quantized. It is only transferred between species in certain sized packets. Because of this we can use absorption and emission of energy to identify species. The energy absorbed by a species is unique because of the unique energy levels for each atom/element/molecule. Spectroscopy involves the absorption and emission of electromagnetic radiation. According to E=hv and c = v, we can calculate the energy absorbed/emitted if we know the wavelength of the radiation. We consider emission spectra or absorption spectra o Absorption: gain in energy to higher energy state. We can determine wavelengths absorbed by looking at the wavelengths that are missing (comparing continuous spectra to transmitted wavelengths) o Emission: gained energy is given off as electron/molecule goes back to ground state. We can see which energies are given off. UV/VIS Spectroscopy Ultraviolet Radiation o Causes changes to higher energy states for the pi electrons in unsaturated molecules o UV spectra are recorded by irradiating the substance with continuously varying UV light. When the wavelength of light corresponds to the amount of energy required to promote a pi electron in an unsaturated molecule to a higher level, energy is absorbed. This absorption is detected and plotted (absorption vs. wavelength). o UV is useful for detecting the presence of double bonds and conjugation o Conjugation occurs when there are multiple double bonds in a molecule that are separated by only one single bond. o Conjugation occurs because of the location of pi-bonds. Recall that pi bonds occur due to axially overplapping p-orbitals. When two pi bonds occur in the same molecule, and close enough to each other, the pi-bonds can “bond” with each other, making a network of pi-bonds which we call a system of conjugation. o The energy absorbed by a pi electron, decreases with increased conjugation. CH2 H2C H2C conjugated CH2 Not conjugated CH2 H2C o o o Energy absorbed by pi-electron decreases with conjugation, 1,3-butadiene absorbs 217 nm, 1,3,5 hexatriene absorbes 258 nm (remember that as wavelength increases, energy decreases) This property can be used to distinguish between isomers because one the conjugated system will absorb UV light at a higher wavelength (lower energy): CH3 CH3 H2C H2C Conjugated 1,3-hexadiene Un-conjugated 1,4-hexadiene When the system of conjugation is extensive, as it is when many benzene rings are attached, the energy absorbed is low enough to be in the visible region. Generally, a molecule with eight or more conjugated double bonds will absorb light in the visible region (400 to 750). This explains the color of chlorophyll, retinol, and indicators like phenolphthalein. The greater the number of conjugated multiple bonds, the longer the wavelength at which the compound absorbs light. O O OPhenolpthalein: O HIn, Clear, transmits all colors O Phenolpthalein: In-, Pink **Notice that it turns pink when the system of conjugation increases so it begins to absorb some wavelengths of visible light and reflect/transmit others, hence the pink color FD & C Blue Food Coloring Based on the demonstration in class, does conjugation increase or decrease for this molecule in increasingly acidic solutions? Molecules called anthocyanins are found in flower petals, leaves, blueberries, blackberries, strawberries, cranberries, cherries, apples and grapes, and in red cabbage. Anthocyanins cause the beautiful red/purple color that you see in roses and in the fall, in maple leaves. Anthocyanins are produced in leaves once chlorophyll (which is green) degenerates in the fall. Nature produces over 300 structurally distinct anthocyanins. By boiling red cabbage leaves, we can take out (extract) the anthocyanins. To get concentrated anthocyanins, cut a head of red cabbage into small pieces (about bite-sized) and boil for about 10 minutes in as little water as necessary to cover the cabbage. Slowly pour (decant) the anthocyanin solution into a jar and store it in the fridge. Anthocyanins dissolved in water (in aqueous solution) change color depending upon the amount of H + and HO- in the water. (That is the same thing as saying that the absorbance of light by anthocyanins depends on pH.) Flowers such as hydrangea contain anthocyanins, and their color is sensitive to the acidity of the soil. The anthocyanins in red cabbage turn bright pink in acid solutions, reddish-purple in neutral solutions and green in basic solutions. But anthocyanins from other plants have other pH/color profiles. The effect of pH on blueberry anthocyanins is shown above.