Part 1, transport in inorganic materials
advertisement

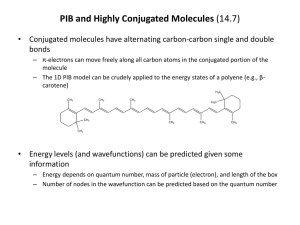
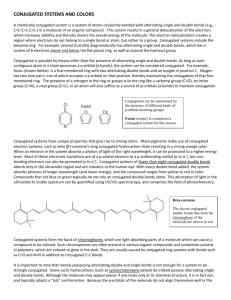
Tentamen i TNE078 Laddningstransport i organiska och oorganiska material (5p) Onsdag den 16 Mars 2005 kl 14.00 - 18.00 Hjälpmedel: engelsk ordbok Examinator: Xavier Crispin Preliminära betygsgränser: tentamen omfattar totalt 38p. För betyg 3 krävs 16p, för betyg 4, 23p, och för betyg 5, 30p. Språk: The English language is recommended. However, if you have any problems write in Swedish. Reminder: Read carefully the questions in order to answer properly and completely! Lycka till, Good luck! Part 1, transport in inorganic materials (20p) 1.1. A simple model of energy bands in semiconductors is the spherical parabolic band model. Give a brief description of it. In the case of silicon, a modification of the model is often made because of the anisotropic effective mass. Describe the modification. (2 p) 1.2. What particles are involved in ionized impurity scattering? When can this scattering mechanism be expected to be important compared to other scattering mechanisms like phonon scattering? How does the scattering rate for ionized impurity scattering depend (qualitatively) on the scattering angle, i e the angle between the momenta of scattered and incident particle? (3 p) 1.3. Consider the Boltzmann transport equation (BTE) in one spatial dimension: f f f f v F t r p t s ( r , p, t ) coll Explain the physical meaning of the function f and make an interpretation of the different terms in the BTE. Would you characterize the equation as classical, quantum mechanical or a mixture? What information is possible to obtain if we have solved the equation and the function f is known? (4 p) 1.4. Describe the relaxation time approximation. Which term in the BTE is approximated and how? (2 p) 1.5. What is a “wide band gap semiconductor”? Give at least two examples of such materials and give some advantages (compared to silicon) and possible applications. (2 p) 1.6. What is the free flight time in a Monte Carlo simulation? Describe at least two different methods of determining the free flight time. (3 p) 1.7. The upper figure shows the hole current as a function of time from a Monte-Carlo simulation of carrier motion in amorphous silicon. The applied voltage was 2 V and the length of the sample 210-4 cm. The temperature was 245 K. Calculate the drift mobility of the holes from the MC data, and compare the value with the experimental value obtained from the lower figure. (4 p) 2 3 Part 2, transport in organic materials (18p) 2.1. Electron transfer reaction: H2 + H*2+ H2+ + H*2 (2p) 2.1.a) Two molecules, H2 and H*2+, are separated by a fixed distance. They undergo an electron transfer reaction. Draw the potential energy curve of the reactants and products versus the reaction coordinate (difference in interatomic distance). 2.1.b) Draw both molecules (bond length) as reactants and products and at the transition state. 2.1.c) From the potential energy curve of one neutral H2 molecule and a positively charged H2+ molecule, define the internal reorganization energy for the electron transfer reaction. 2.2. Electron transfer and charge transport (3p) In an un-doped organic crystal at room temperature, the charge transport usually occurs in a hopping regime between adjacent molecules. In other words, the fundamental event of charge hopping is a self-exchange electron transfer reaction. In the semi-classical model, the rate of this reaction is given by the formula: 2.2.a) What are T, t and λ? Explain their physical meaning. 2.2.b) How does “t” depend on the intermolecular distance? 2.2.c) What type of morphology and crystal structure would give rise to the largest charge carrier mobility? 2.3. Charge transport in neutral conjugated materials (3p) 2.3.a) What is the order of magnitude of the electrical conductivity in un-doped conjugated polymers? 2.3.b) Does the temperature affect the charge carrier density in un-doped conjugated polymers? Why? 2.3.c) Give and explain one criterion to estimate if charge carriers travel in a hopping regime or a band motion regime? 2.3.d) In the band motion and hopping regimes, the charge carrier mobility has different temperature dependence. What are they? 2.3.e) What is a polaron in a conjugated polymer? Sketch the structure of a polaron for a conjugated polymer of your choice. 4 2.4. Doped conjugated polymers (3p) 2.4.a) What is ”variable range hopping-VRH” theory? 2.4.b) For what kind of materials is the VRH theory used? 2.4.c) What is Mott’s law? Give it 2.4.d) How can one increase the electrical conductivity of polyaniline in its emeraldine base form? 2.4.e) How is the conductivity vs. temperature dependence affected by increasing the disorder in a polyaniline layer? 2.5. Ionic transport (3p) The 85th edition of the CRC Handbook of Chemistry and Physics gives values of the diffusion coefficient D in water for Cl- and Na+ of 2.03210-5 cm2/sec and 1.33410-5 cm2/sec, respectively. 2.5.a) What is the mobility µ of Na+ in water at room temperature (293K)? Of Cl-? What is the ionic conductivity of a 0.01M aqueous solution of NaCl? (1M = 1 mol/liter) 2.5.b) At equilibrium with no applied field, the concentrations will be uniform and equal everywhere in the above electrolyte. What happens near a metal electrode with an applied potential of -1V? Assume that the potential in the electrolyte far away from the electrode is 0V and the concentrations are both 0.01M. Sketch the concentration profiles of each of the above species and the potential within the electrolyte versus the distance from the electrode. It is OK to approximate the ions as point charges with no volume. 2.5.c) About how close to the electrode must you look to observe concentrations and/or potential that is different from that observed in the bulk? An order of magnitude is OK, but state where the estimate comes from. 2.6. Doping and electrochemistry (2p) Chemists and physicists often use different language to describe the same thing. For example, chemists talk about oxidation and reduction, while physicists talk about doping and dedoping. 2.6.a) Write a chemical half-reaction for a conjugated polymer (generically or for a specific material) and indicate whether the half-reaction you wrote is a reduction or an oxidation. 2.6.b) Is the reaction you wrote above a doping or a dedoping process? Which is the more electronically conductive species? Which is the doped species? Which is the neutral species? 5 2.7. Electrochemical device structure and behavior (2p) 2.7.a) Sketch a lateral (both anode and cathode in the same plane with electrolyte on top) structure 2 device and indicate how to apply an electric potential. Clearly indicate where the conducting polymer and the electrolyte are, and which electrode will be the anode and which will be the cathode when a potential is applied in the direction you indicate. 2.7.b) When a potential is applied to such a device made out a conjugated polymer (like PEDOT:PSS), a color change is observable. This is called electrochromism. What color does each side become in a PEDOT:PSS device? If the electrolyte is a poor conductor (few ions, low mobility), which part of each electrode will switch first? A sketch may be appropriate. 6