Methodological Challenges
advertisement

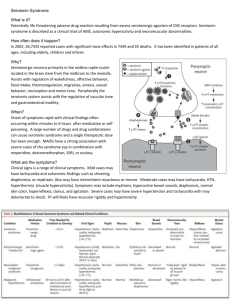
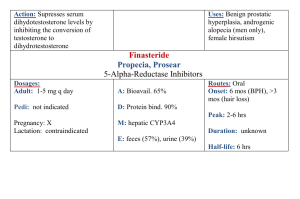
Identifying the Role of Serotonin in Cognitive Dysfunction in Breast Cancer Survivors: Methodological Challenges Diane M. Von Ah, PhD, RN & Janet S. Carpenter, PhD, RN MNRS Research Brief Women’s Health Section Background Although cognitive dysfunction is a prevalent and disruptive problem for many breast cancer survivors (BCS), little research has examined its etiology. One potential mechanism that remains to be explored is serotonin. Serotonin receptors are located in brain areas implicated in normal and dysfunctional cognitive processes and serotonin levels are significantly affected by estrogen withdrawal—a common side effect of breast cancer treatment. However, no study has evaluated serotonin’s impact on cognitive dysfunction in BCS. Exploration of the underlying etiology of cognitive dysfunction in BCS will help determine the efficacy of developing interventions that target the central serotonin system either behaviorally (e.g., diet) or pharmacologically. However, the complexity of this type of physiological research may also present methodological challenges for nurse scientists. Research in Progress The purpose of this pilot study was to examine the role of serotonin in cognitive dysfunction in BCS by lowering central serotonin concentrations via acute tryptophan depletion. This was a within-subjects, double blind, placebo controlled, crossover study. Female BCS who were >1 month but < 5 years post-treatment (surgery, radiation, chemotherapy) for non-metastatic breast cancer were recruited. Consenting participants received acute tryptophan depletion based on published procedures or a control condition in random order during two test days at the General Clinical Research Center. On one day, participants ingested a concentrated amino acid drink and encapsulated amino acids (no tryptophan) according to published procedures that have been shown to have specific effects on serotonin within 4.5 to 7 hours. On the other day, women ingested a control drink with no effects on serotonin. Serial venous blood sampling was used to monitor response to each condition and to investigate genetic polymorphisms that could affect response. Cognitive dysfunction was measured at the same time each test day using standardized neuropsychological tests. Data will be analyzed using descriptive statistics, MANOVA for overall differences between groups and MANCOVA to adjust for potential covariates such as age, education, previous medical treatment and current medications. Our main hypothesis will be supported if cognitive dysfunction is exacerbated during acute tryptophan depletion. Methodological Challenges Two methodological challenges associated with this type of complex physiological research include costs per patient enrolled and consented to the study and addressing issues of blinding subjects and testers (data collectors) when using this type of placebo cross-over design. A total of 36 patients consented to the study. We estimated patient-related costs by totaling expenditures for staff (project manager, data collectors, and recruiters) and supplies and dividing this total by the number of patients consented to the study. The total cost per patient enrolled was $3,900. This total did not include in-kind support provided by the General Clinical Research Center which included dietician time to prepare the amino acids, nurse monitoring of patients, costs associated with hourly blood draws, and room and meal costs. Second, details of blinding subjects and research staff are an important consideration for this type of study design. A thorough protocol stipulating procedures to ensure blinding was developed prior to the implementation of this study. The protocol detailed names and titles of staff who would and would not be blinded. Methods to ensure blinding on both test days included preventing subjects and staff from seeing the drink contents (metal cup with opaque lid); masking the taste of the drink with a flavoring of the subjects’ choice; providing an equal number of encapsulated amino acids; and following the exact study protocol including hourly blood draws and routine safety monitoring. Our experience with recruitment and enrollment costs and blinding procedures will help inform our plans for a larger study exploring the role of serotonin in cognitive dysfunction in breast cancer survivors. This study was funded by the ONS Foundation Postdoctoral Fellowship Award (RE02, Von Ah); Indiana University General Clinical Research Center, CReFF Program Award (Von Ah); Postdoctoral Fellowship, Grant #T32NR007066 from NINR/NIH, to Indiana University, School of Nursing, Indianapolis (Von Ah) and the Department of Defense Breast Cancer Program (BC043199, Carpenter). Diane Von Ah is an Assistant Professor and Robert Wood Johnson Foundation Nurse Faculty Scholar at Indiana University School of Nursing, Department of Adult Health. Diane Von Ah can be contacted at dvonah@iupui.edu